Western blotting, also known as immunoblotting, is a widely used laboratory technique in molecular biology and biochemistry. It is employed to detect and analyze specific proteins in a complex mixture of proteins, such as those extracted from cells or tissues. Western blotting involves several steps and uses antibodies to target and detect specific proteins of interest.
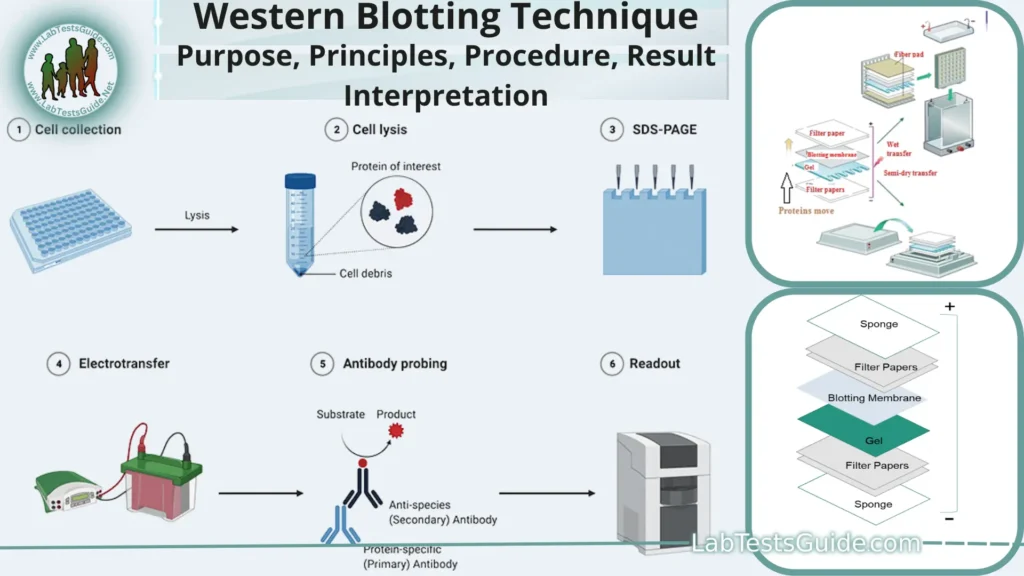
Western blotting, also known as protein immunoblot, is a laboratory technique used to detect specific proteins in a sample of tissue homogenate or extract. It is a widely used analytical technique in molecular biology and immunogenetics.
Key points of Western Blotting:
- Purpose: Identifying and analyzing specific proteins in a sample.
- Sample Prep: Extract, denature, and load proteins onto a gel.
- Electrophoresis: Separate proteins by size using SDS-PAGE.
- Transfer: Move proteins from gel to a membrane.
- Blocking: Prevent nonspecific antibody binding.
- Primary Antibody: Bind to target protein on the membrane.
- Washing: Remove unbound primary antibodies.
- Secondary Antibody: Binds to primary antibody.
- Washing: Remove unbound secondary antibodies.
- Detection: Visualize the target protein signal.
- Quantification: Measure signal intensity for protein levels.
- Controls: Use positive and negative controls.
- Molecular Weight Marker: Estimate protein sizes.
- Antibody Specificity: Ensure antibodies are specific to the target.
- Background Reduction: Minimize nonspecific binding.
- Quality Control: Confirm proper electrophoresis and transfer.
- Data Analysis: Analyze results for protein insights.
- Applications: Used in research and diagnostics.
Defination of Western Blotting:
Western Blotting, also known as immunoblotting, is a laboratory technique used to detect and analyze specific proteins in a complex mixture of proteins extracted from cells or tissues. It involves separating proteins by size through gel electrophoresis, transferring them to a membrane, and then using antibodies to detect and visualize the target protein.
Background and Significance:
- Protein Analysis: Western blotting is a fundamental method for studying protein expression and characteristics.
- Disease Research: It is crucial in biomedical research, aiding in the investigation of diseases and biomarker discovery.
- Pharmacology: Western blots help evaluate drug effects on proteins and assess treatment outcomes.
- Clinical Diagnostics: Used in clinical labs to detect specific disease-related proteins.
- Molecular Biology: Essential for studying protein post-translational modifications and interactions.
- Quality Control: Common in biotechnology and pharmaceutical industries for quality assurance.
- Personalized Medicine: Contributes to personalized treatment approaches based on protein profiles.
- Research Advancement: Continues to advance our understanding of cell biology and disease mechanisms.
Purpose of Western Blotting:
- Protein Identification: To identify and confirm the presence of a particular protein of interest in a biological sample.
- Protein Quantification: To measure the relative abundance or expression levels of a specific protein in different samples.
- Post-Translational Modification Analysis: To study and characterize post-translational modifications (e.g., phosphorylation, glycosylation) of proteins.
- Protein Size Determination: To estimate the molecular weight of proteins by comparing their migration on a gel to molecular weight markers.
- Protein-Protein Interaction Studies: To investigate protein interactions and complexes by detecting co-immunoprecipitated proteins.
- Biomarker Discovery: To identify potential biomarkers associated with diseases or specific physiological conditions.
- Quality Control: In biotechnology and pharmaceutical industries to assess the purity and integrity of protein samples.
- Clinical Diagnostics: In medical laboratories to diagnose diseases or monitor disease progression by detecting specific disease-related proteins.
- Drug Development: To assess the effects of drugs or therapies on target proteins and pathways.
- Cell Signaling Pathway Analysis: To investigate the activation or inhibition of signaling pathways by monitoring changes in protein expression and phosphorylation.
Applications of Western Blotting:
- Protein Detection: Identify specific proteins in complex mixtures.
- Protein Quantification: Measure protein levels in samples.
- Post-Translational Modification Analysis: Study protein modifications.
- Molecular Weight Determination: Estimate protein sizes.
- Protein-Protein Interaction Studies: Investigate protein complexes.
- Biomarker Discovery: Identify disease-related markers.
- Quality Control: Assess protein purity in biotech and pharma.
- Clinical Diagnostics: Diagnose diseases via protein detection.
- Drug Development: Evaluate drug effects on target proteins.
- Cell Signaling Analysis: Monitor signaling pathway changes.
Principles of Western Blotting:
- Protein Separation: The technique begins with the separation of proteins from a biological sample based on their size using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS helps denature proteins and ensures they migrate primarily based on molecular weight.
- Transfer: After electrophoresis, the separated proteins are transferred from the gel onto a solid membrane, typically made of nitrocellulose or polyvinylidene fluoride (PVDF). This transfer is achieved through a process known as electroblotting.
- Blocking: To prevent nonspecific binding of antibodies, the membrane is treated with a blocking solution. Common blocking agents include milk, bovine serum albumin (BSA), or non-fat dry milk. Blocking reduces background signal.
- Primary Antibody Incubation: The membrane is incubated with a primary antibody that is specific to the target protein of interest. The primary antibody binds to the target protein.
- Washing: Unbound primary antibodies are washed away to reduce background noise and ensure specificity.
- Secondary Antibody Incubation: A secondary antibody is applied, which is conjugated to an enzyme (e.g., horseradish peroxidase) or a fluorophore. The secondary antibody binds specifically to the primary antibody.
- Washing: Unbound secondary antibodies are washed away.
- Detection: The target protein is visualized by introducing a substrate for the enzyme linked to the secondary antibody (if an enzyme is used) or by using a fluorescence detector (if a fluorophore is used). This produces a signal that corresponds to the presence and amount of the target protein.
- Quantification: The intensity of the bands representing the target protein on the membrane is measured, usually via densitometry, to quantify the protein’s expression level.
- Controls: Positive and negative controls are used to validate the experiment. The positive control contains the target protein, while the negative control lacks it.
- Molecular Weight Marker: Molecular weight markers are run alongside the protein samples to estimate the molecular weight of the detected proteins.
- Antibody Specificity: It is crucial to ensure that both primary and secondary antibodies are specific to their respective targets to avoid false results.
- Data Analysis: The data obtained from the Western blot is analyzed to interpret protein expression, modifications, or interactions within the sample.
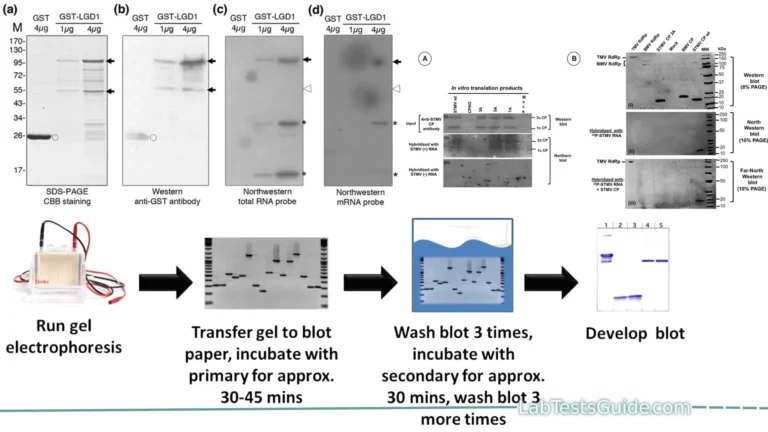
Procedure for Western Blotting:
The Western blotting procedure involves several steps to detect and analyze specific proteins in a biological sample. Here is a general outline of the Western blotting procedure:
Materials and Reagents:
- Protein samples
- SDS-PAGE gel
- Transfer buffer
- Nitrocellulose or PVDF membrane
- Blocking buffer (e.g., milk or BSA)
- Primary antibody specific to the target protein
- Secondary antibody conjugated to an enzyme or fluorophore
- Wash buffer
- Detection reagent (e.g., chemiluminescent substrate for enzyme-conjugated antibodies)
- Molecular weight marker
- Electrophoresis and transfer equipment
- Western blotting apparatus
Procedure:
- Sample Preparation:
- Extract proteins from your biological sample using an appropriate method.
- Denature the proteins by heating them in a sample buffer containing SDS and a reducing agent (e.g., β-mercaptoethanol).
- Gel Electrophoresis:
- Load the denatured protein samples onto an SDS-PAGE gel according to their molecular weight.
- Run electrophoresis at a constant voltage until proteins have separated based on size.
- Protein Transfer:
- Transfer the separated proteins from the gel to a nitrocellulose or PVDF membrane using a transfer apparatus. This is typically done by electroblotting.
- Blocking:
- Incubate the membrane in a blocking buffer to prevent nonspecific binding of antibodies. This step typically takes about an hour.
- Primary Antibody Incubation:
- Incubate the membrane with a primary antibody specific to the target protein overnight or for several hours at 4°C or at room temperature.
- Washing:
- Wash the membrane several times with a wash buffer to remove unbound primary antibodies.
- Secondary Antibody Incubation:
- Incubate the membrane with a secondary antibody that is conjugated to an enzyme or fluorophore. This secondary antibody binds specifically to the primary antibody and is used for detection.
- Washing:
- Wash the membrane again to remove unbound secondary antibodies.
- Detection:
- Visualize the target protein by adding a detection reagent suitable for the secondary antibody label. For enzyme-linked secondary antibodies, this typically involves adding a chemiluminescent substrate and exposing the membrane to X-ray film. For fluorophore-conjugated antibodies, use a fluorescence detector.
- Quantification:
- Quantify the intensity of the protein bands using imaging software or a gel documentation system. This provides information about the protein’s expression level.
- Controls:
- Include positive and negative controls to validate the experiment. The positive control should contain the target protein, while the negative control should lack it.
- Molecular Weight Marker:
- Use the molecular weight marker bands on the gel to estimate the molecular weight of the detected proteins.
- Data Analysis:
- Analyze the Western blot data to interpret protein expression, modifications, or interactions within the sample.
Result Interpretation:
- Identification of Protein Bands:
- Identify the protein bands on the membrane. Each band represents a specific protein or protein isoform.
- Compare the positions of these bands to the molecular weight marker bands to estimate the molecular weight of the detected proteins.
- Positive and Negative Controls:
- Check the results of your positive and negative controls. The positive control should show the expected band(s) for the target protein, while the negative control should have no band in that position.
- Protein Expression Levels:
- Assess the intensity of the protein bands. Stronger bands indicate higher protein expression levels in the sample.
- Quantify the band intensity using image analysis software, if necessary, to obtain semi-quantitative data.
- Post-Translational Modifications (PTMs):
- If you’re studying PTMs, look for shifts in the molecular weight of the bands compared to the unmodified protein. This can indicate the presence of modified forms of the protein.
- Multiple Bands for One Protein:
- Sometimes, a single protein can produce multiple bands due to alternative splicing or PTMs. Analyze the multiple bands and consider their significance.
- Background Noise:
- Ensure that there is minimal background noise or unspecific binding, which can interfere with interpretation. Proper blocking and washing steps help reduce background.
- Replicates and Consistency:
- If you have replicates, check for consistency in band patterns between replicates. This increases the reliability of your results.
- Comparative Analysis:
- Compare the Western blot results of different samples or conditions to draw conclusions about changes in protein expression or modifications under different experimental conditions.
- References and Literature:
- Refer to published studies or literature for expected results and benchmarks. Compare your findings with established knowledge.
- Data Validation:
- Validate your results with complementary techniques like RT-qPCR, mass spectrometry, or immunoprecipitation to confirm the presence or absence of specific proteins or PTMs.
- Statistical Analysis:
- If applicable, perform statistical analysis to determine if observed differences in band intensity between samples are statistically significant.
- Biological Context:
- Consider the biological context of your experiment. Interpret the results in the context of your research objectives and hypotheses.
- Conclusion:
- Based on the above considerations, draw conclusions about the presence, abundance, and characteristics of the target protein(s) in your sample.
Remember that Western blotting is a semi-quantitative technique, and the interpretation should take into account the limitations and potential sources of variability. Careful experimental design, proper controls, and thorough analysis are essential for accurate result interpretation.
Troubleshooting and Tips:
- Weak or No Bands:
- Check the protein loading amount.
- Verify the efficiency of protein transfer.
- Ensure antibody specificity.
- High Background Noise:
- Improve blocking (use fresh blocking buffer).
- Optimize antibody dilutions.
- Enhance washing steps.
- Non-Specific Binding:
- Use a blocking agent appropriate for your detection system.
- Ensure antibody specificity and validation.
- Smiling Bands:
- Overloaded lanes can cause this; load less protein.
- Uneven Bands:
- Ensure even gel loading.
- Check for air bubbles during gel casting.
- Multiple Bands for One Protein:
- Consider protein isoforms or post-translational modifications.
- High Molecular Weight Bands Smear:
- Verify gel concentration; use a lower percentage gel.
- Low Signal Intensity:
- Use a more sensitive detection method.
- Optimize antibody concentrations.
- Non-Reproducible Results:
- Standardize protocols and use consistent reagents.
- Carefully handle gels and membranes.
- Check Antibody Freshness:
- Ensure antibodies are not expired.
- Positive and Negative Controls:
- Always include controls to validate results.
- Experimental Replication:
- Perform replicates to enhance result reliability.
- Documentation and Analysis:
- Maintain detailed records and use quantitative analysis.
- Use Molecular Weight Markers:
- Include markers for molecular weight estimation.
- Optimize Gel Electrophoresis:
- Ensure proper running conditions.
- Quality Sample Preparation:
- Maintain protein integrity during extraction.
- Be Patient and Diligent:
- Western blotting can require optimization; don’t hesitate to troubleshoot.
- Consult Literature and Colleagues:
- Seek advice from experienced researchers and published protocols.
Advantages and Disadvantages of Western Blotting:
Advantages of Western Blotting:
- Specific Protein Detection: Western blotting allows for the specific detection of individual proteins in a complex mixture.
- Protein Quantification: It provides semi-quantitative information about protein expression levels.
- Post-Translational Modification Analysis: Western blotting can identify and characterize protein post-translational modifications.
- Versatility: It can be applied to various sample types, including cells, tissues, and bodily fluids.
- Protein-Protein Interaction Studies: Useful for investigating protein-protein interactions and complexes.
- Biomarker Discovery: Aids in the discovery of disease-related biomarkers.
- Quality Control: Commonly used in biotechnology and pharmaceutical industries for quality assurance.
- Clinical Diagnostics: Employed in medical labs for disease diagnosis and monitoring.
Disadvantages of Western Blotting:
- Semi-Quantitative: It provides relative, not absolute, protein quantification.
- Labor-Intensive: The procedure involves several steps and can be time-consuming.
- Technique Variation: Results can vary based on variations in technique and reagents.
- Subjectivity: Interpretation may be subjective, especially for weak or multiple bands.
- Background Noise: High background noise can occur, affecting data quality.
- Limited Multiplexing: Typically, only one protein per blot can be analyzed, limiting multiplexing capabilities.
- Expensive Reagents: Antibodies and detection reagents can be costly.
- Skill and Experience: Requires expertise for optimization and reliable results.
- Data Analysis: Quantification and analysis may require specialized software.
Limitations of Western Blotting:
- Semi-Quantitative: Provides relative, not absolute, quantification of protein levels.
- Labor-Intensive: Involves multiple steps and can be time-consuming.
- Variability: Results can vary based on variations in technique and reagents.
- Subjectivity: Interpretation may be subjective, especially for weak or multiple bands.
- Background Noise: High background noise can affect data quality.
- Limited Multiplexing: Typically, only one protein per blot can be analyzed.
- Expensive Reagents: Antibodies and detection reagents can be costly.
- Skill and Expertise: Requires expertise for optimization and reliable results.
- Data Analysis: Quantification and analysis may require specialized software.
- Inefficiency for Low-Abundance Proteins: May not detect proteins with low expression levels.
- Cross-Reactivity: Antibodies can cross-react with unintended proteins.
- Validation Challenges: Proper antibody validation is crucial for specificity.
Variations and Modern Alternatives:
Variations of Western Blotting:
- Semi-Dry Blotting: A faster and more economical variation of electroblotting.
- Tank Blotting: An alternative electroblotting method.
- Multiplex Western Blotting: Simultaneously detects multiple proteins in a single blot.
- Dot Blotting: Used for qualitative protein detection, primarily for screening purposes.
- Slot Blotting: Similar to dot blotting but for larger sample volumes.
- Capillary Blotting: Uses capillaries to transfer proteins to membranes.
Modern Alternatives to Western Blotting:
- Mass Spectrometry (MS): Provides accurate protein identification and quantification.
- Enzyme-Linked Immunosorbent Assay (ELISA): Quantifies proteins with high specificity and sensitivity.
- Reverse Phase Protein Array (RPPA): High-throughput analysis of protein expression in samples.
- Protein Microarrays: Enable simultaneous analysis of multiple proteins for protein-protein interactions, biomarker discovery, and more.
- Immunohistochemistry (IHC): Used for protein detection and localization in tissue sections.
- Proximity Ligation Assay (PLA): Detects protein-protein interactions with high specificity.
- Selected Reaction Monitoring (SRM) and Multiple Reaction Monitoring (MRM): Mass spectrometry-based quantitative methods for specific protein targets.
- Flow Cytometry: Analyzes proteins at the single-cell level, useful for cell surface and intracellular protein detection.
- Single-Cell Western Blotting: Emerging technique for protein analysis in single cells.
- Single-Molecule Detection Techniques: Techniques like Single-Molecule Fluorescence In Situ Hybridization (smFISH) and Single-Molecule Localization Microscopy (SMLM) offer high-resolution protein detection.
- Digital PCR (dPCR): Quantifies DNA or RNA indirectly through PCR, but can be adapted for protein detection through immuno-dPCR.
- Proximity Extension Assay (PEA): A high-throughput method for protein detection and quantification in biological samples.
- Chemiluminescent Western Blot Imaging Systems: Advanced imaging systems for increased sensitivity and quantitative analysis.
- Automated Western Blotting Systems: High-throughput platforms that automate many Western blotting steps for efficiency and reproducibility.
Comparison of Western Blotting with Modern Techniques:
| Aspect | Western Blotting | Mass Spectrometry (MS) | Enzyme-Linked Immunosorbent Assay (ELISA) | Reverse Phase Protein Array (RPPA) | Protein Microarrays |
|---|---|---|---|---|---|
| Protein Detection | Specific | Highly specific | Highly specific | Specific | Highly specific |
| Quantitative Capability | Semi-quantitative | Quantitative | Quantitative | Quantitative | Quantitative |
| High-Throughput | Limited | Limited | High-throughput | High-throughput | High-throughput |
| Multiplexing | Limited (multiplex WB) | Limited | High (multiplex ELISA) | High (multiple proteins) | High (multiple proteins) |
| Sensitivity | Variable, can be low | High | High | Variable | Variable |
| Sample Amount Required | Moderate to high | Low | Low | Low | Low |
| Specificity and Cross-Reactivity | Possible cross-reactivity | High specificity | High specificity | High specificity | High specificity |
| Protein Modifications Detection (e.g., PTMs) | Yes, with optimization | Yes, with specialized MS | Limited | Limited | Limited |
| Data Complexity | Moderate to low | High | Moderate | High | High |
| Data Analysis | Manual or software tools | Specialized software | Software analysis | Specialized software | Specialized software |
| Time Required | Hours to a day | Hours to days | Hours to a day | Hours to days | Hours to days |
| Cost | Moderate to high | High | Moderate | Moderate | High |
| Common Applications | Research, diagnostics | Proteomics, biomarker ID | Clinical diagnostics, research | Protein profiling, biomarkers | Protein interaction, research |
FAQs:
1. What is Western blotting used for?
- Western blotting is used to detect and analyze specific proteins in a complex mixture, providing information about protein expression levels, post-translational modifications, and protein-protein interactions.
2. How does Western blotting work?
- Western blotting involves separating proteins by size through SDS-PAGE, transferring them to a membrane, and using antibodies to detect the target protein of interest.
3. What is the difference between Western blotting and ELISA?
- ELISA (Enzyme-Linked Immunosorbent Assay) is a plate-based technique for quantifying proteins, while Western blotting is used for protein detection and characterization. ELISA is generally more quantitative, while Western blotting provides qualitative and semi-quantitative data.
4. What are the key steps in a Western blot protocol?
- The key steps include sample preparation, gel electrophoresis, protein transfer, blocking, primary and secondary antibody incubation, washing, detection, and data analysis.
5. How do I choose the right antibodies for Western blotting?
- Select antibodies with high specificity to the target protein and consider factors like antibody source, validation, and dilution. Validation with positive and negative controls is essential.
6. What can cause high background in Western blots?
- High background can result from improper blocking, inadequate washing, non-specific binding of antibodies, or the use of deteriorated reagents.
7. Can Western blotting be quantitative?
- Western blotting can provide semi-quantitative data by comparing band intensities. However, it is not as quantitative as techniques like mass spectrometry.
8. What are some common troubleshooting tips for Western blotting?
- Troubleshooting tips include optimizing antibody concentrations, checking protein loading, ensuring efficient protein transfer, and validating antibody specificity.
9. What are some modern alternatives to Western blotting?
- Modern alternatives include mass spectrometry (MS), enzyme-linked immunosorbent assay (ELISA), reverse phase protein array (RPPA), protein microarrays, and proximity ligation assay (PLA).
10. Can Western blotting be used in clinical diagnostics?
- Yes, Western blotting is used in clinical labs for diagnosing diseases and monitoring disease progression by detecting specific disease-related proteins.
11. Is there a way to multiplex Western blotting to detect multiple proteins at once?
- Yes, variations of Western blotting, such as multiplex Western blotting, allow for the simultaneous detection of multiple proteins on the same membrane using different antibodies and detection methods.
Conclusion:
In conclusion, Western blotting, also known as immunoblotting, is a widely used laboratory technique that plays a crucial role in protein analysis and research. It enables the specific detection and analysis of proteins in complex biological samples. Western blotting provides valuable insights into protein expression levels, post-translational modifications, and protein-protein interactions.
While Western blotting has been a staple in molecular biology and biochemistry for decades, it does have limitations, including its semi-quantitative nature, subjectivity in interpretation, and the potential for background noise. Researchers must carefully design experiments, optimize protocols, and validate antibodies to ensure the reliability of Western blot results.
Home | Blog | About Us | Contact Us | Disclaimer
Possible References Used




4 Comments