Researchers at Stanford Medicine have achieved a groundbreaking milestone by reconstructing a key human nerve pathway responsible for pain sensation in a lab dish. This innovation could revolutionize pain research and lead to more effective treatments for chronic pain, a condition affecting over 116 million Americans.
The study was published in the journal Nature.
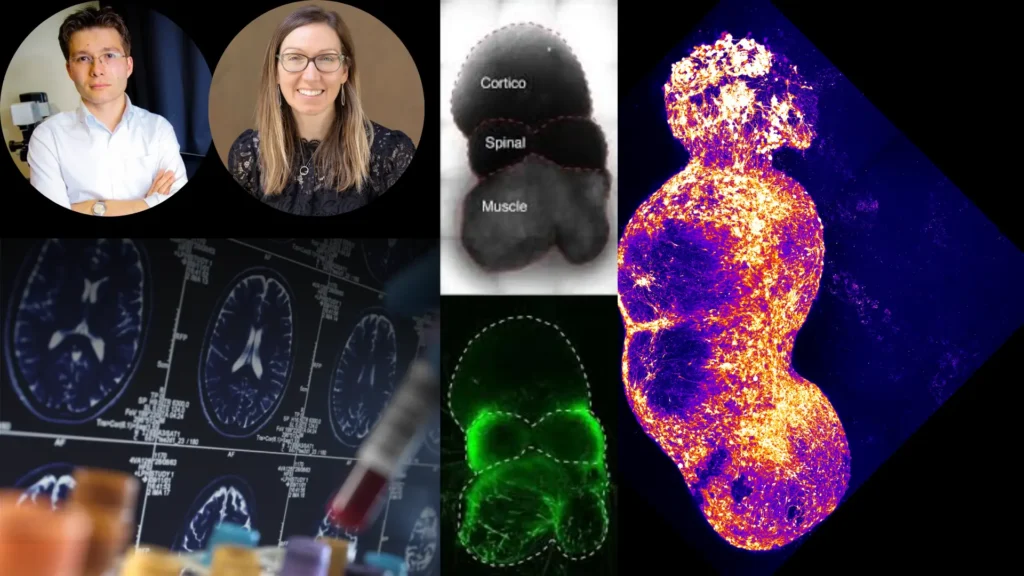
Published today in Nature1, the study describes how scientists assembled four miniaturized components of the human nervous system to replicate the ascending sensory pathway—the critical circuit that transmits pain signals from the skin to the brain.
A Breakthrough in Pain Research
Led by Dr. Sergiu Pasca2, professor of psychiatry and behavioral sciences, the team successfully modeled the pathway using lab-grown neural organoids3, tiny 3D clusters of human cells that mimic brain and nerve tissue. By connecting organoids representing the dorsal root ganglion, spinal cord, thalamus, and somatosensory cortex, they recreated the biological relay system that conveys pain.
“We can now model this pathway non-invasively,” said Pasca. “This will help us understand pain disorders and develop better treatments.”
Why This Matters
Chronic pain is notoriously difficult to study in animals because their nervous systems differ from humans’. Traditional painkillers, such as opioids, are often addictive and ineffective for long-term relief.
Dr. Vivianne Tawfik4, a pain specialist not involved in the study, emphasized the urgency of new treatments: “It’s heartbreaking to see patients suffer when we’ve exhausted all options. This research offers hope.”
How They Did It
The team used induced pluripotent stem cells (iPSCs)—derived from human skin samples—to grow organoids representing each part of the pain pathway. Over 100 days, these organoids fused into a functional circuit, dubbed an “assembloid”, which exhibited synchronized electrical activity resembling real pain signaling.
Key findings:
- Capsaicin (the compound in chili peppers) triggered pain-like signals in the assembloid.
- Mutations in the Nav1.7 sodium channel5, linked to extreme pain sensitivity, caused hyperactive signaling.
- Disabling Nav1.7 disrupted pain signal coordination, suggesting new drug targets.
Future Applications
This assembloid model could:
✔ Screen non-addictive pain drugs targeting only peripheral nerves.
✔ Study autism-related sensory hypersensitivity, as some autism genes affect pain pathways.
✔ Help researchers understand chronic pain mechanisms at a cellular level.
Stanford has filed a patent for this technology, marking a major step toward personalized pain therapies.
- Human assembloid model of the ascending neural sensory pathway – Nature – (Accessed on Apr 11, 2025) ↩︎
- Sergiu P. Pasca – Professor of Psychiatry and Behavioral Sciences – Sleep Medicine – (Accessed on Apr 11, 2025) ↩︎
- After Months In A Dish, Lab-Grown Minibrains Start Making ‘Brain Waves’ – nrp.org – (Accessed on Apr 11, 2025) ↩︎
- Vivianne Tawfik – Associate Professor – University Medical Line, Anesthesiology, Perioperative and Pain Medicine – (Accessed on Apr 11, 2025) ↩︎
- Non-opioid proves effective for acute surgical & non-surgical pain – New Atlas – (Accessed on Apr 11, 2025) ↩︎
References
- Stanford Scientists Recreate Human Pain Pathway in a Dish, Paving the Way for Better Treatments – Stanford Medicine – (Accessed on Apr 10, 2025)
- Pain pathway in a dish could aid search for new analgesic drugs – nrp.org – (Accessed on Apr 11, 2025)
Possible References Used