Spectrophotometry is a key analytical technique used in clinical laboratories for measuring the concentration of analytes in biological samples such as blood, urine, and other body fluids. It is commonly used for assays including glucose, cholesterol, hemoglobin, and enzyme activities.
Introduction
Spectrophotometry is a key analytical technique used in clinical laboratories for measuring the concentration of analytes in biological samples such as blood, urine, and other body fluids. It is commonly used for assays including glucose, cholesterol, hemoglobin, and enzyme activities.
Principle
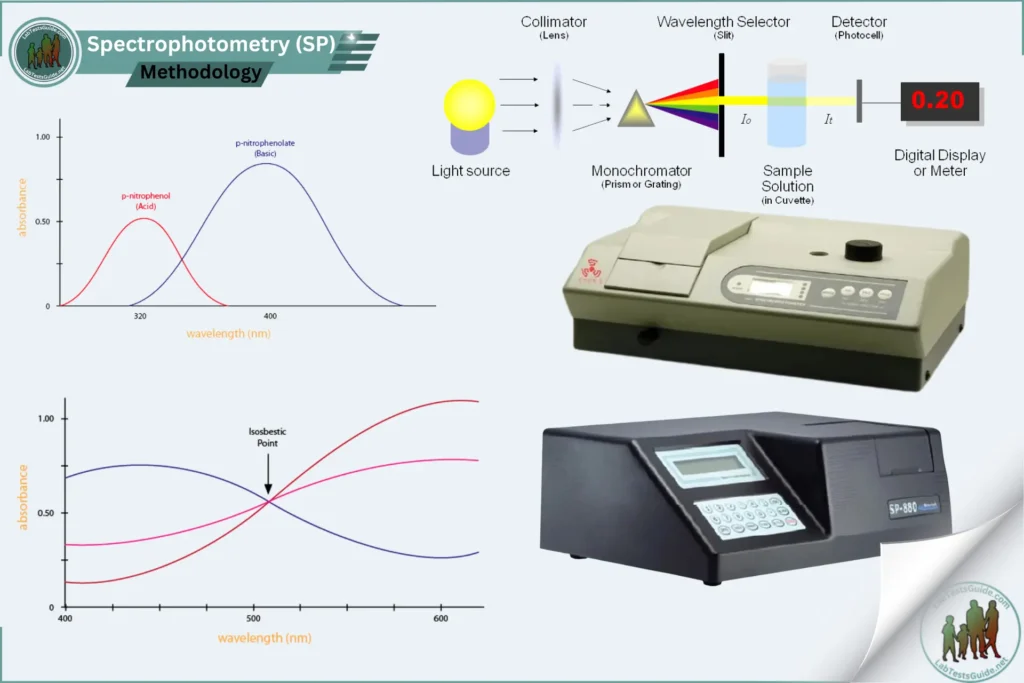
Spectrophotometry involves measuring the absorbance of light by a sample at a specific wavelength. The absorbance is directly proportional to the concentration of the analyte, according to Beer-Lambert’s law.
Specimen Requirements
- Type of specimen: Blood, serum, plasma, urine, or other body fluids
- Volume of specimen required: Typically 200-500 µL
- Collection method: Standard venipuncture for blood, clean-catch midstream for urine
- Handling and storage requirements: Store blood samples at 2-8°C if not analyzed immediately, urine samples should be analyzed promptly or refrigerated
- Stability of the specimen: Dependent on the analyte and the storage conditions
Reagents and Materials
- Diluent or buffer solution appropriate for the specific assay
- Calibration standards with known concentrations of the analyte
- Control samples (low, normal, high)
- Reaction reagents specific to the test (e.g., glucose oxidase for glucose measurement)
- Cuvettes (quartz or plastic, depending on wavelength requirements)
- Distilled water
Equipment
- Spectrophotometer capable of measuring the desired wavelength
- Cuvettes compatible with the spectrophotometer
- Pipettes and tips
- Calibration standards for the assay
- Centrifuge (if serum or plasma is needed)
Procedure
Preparation:
- Turn on the spectrophotometer and allow it to warm up.
- Select the appropriate wavelength for the analyte (e.g., 340 nm for NADH assays, 500 nm for glucose oxidase assays).
- Prepare a blank solution containing only the diluent or buffer. Calibration:
- Prepare a series of calibration standards with known concentrations of the analyte.
- Measure the absorbance of each standard at the selected wavelength.
- Plot the absorbance versus concentration to create a standard curve. Sample Measurement:
- Prepare the sample by mixing it with the appropriate reaction reagents.
- If necessary, centrifuge blood samples to obtain serum or plasma.
- Rinse the cuvette with distilled water and then with a small amount of the sample.
- Fill the cuvette with the sample and place it in the spectrophotometer.
- Measure the absorbance of the sample at the selected wavelength.
- Record the absorbance reading.
Quality Control
- Ensure the spectrophotometer is calibrated correctly before use.
- Run blank, calibration standards, and control samples to verify accuracy and precision.
- Include internal quality control samples in each batch of tests.
Calculation and Interpretation
- Calculate the concentration of the analyte in the sample using the standard curve equation.
- Compare the results with reference ranges provided for the specific test.
- Consider potential sources of error, such as hemolysis, lipemia, or icterus in blood samples.
Limitations
- Spectrophotometry may be affected by interfering substances such as lipids, hemoglobin, or bilirubin.
- Proper calibration and maintenance of the spectrophotometer are essential for accurate results.
- High or low concentrations of analytes may require sample dilution to fall within the linear range of the assay.
Safety Precautions
- Wear appropriate PPE (gloves, lab coat, safety goggles).
- Handle all reagents and samples according to standard biohazard protocols.
- Dispose of waste according to local regulations.
References
- Clinical Chemistry: Principles, Techniques, and Correlations by Michael L. Bishop, Edward P. Fody, and Larry E. Schoeff.
- Tietz Textbook of Clinical Chemistry and Molecular Diagnostics.
- Manufacturer’s instructions for spectrophotometer operation and assay kits.
- Clinical Laboratory Standards Institute (CLSI) guidelines.
- Atkins, Peter and Julio de Paula. Physical Chemistry for the Life Sciences. New York: Oxford University Press, 2006.
- Chang, Raymond. Physical Chemistry for the Biosciences. USA: University Science Books, 2005.
- Gore, Michael. Spectrophotometry & Spectrofluorimetry. New York: Oxford University Press, 2000.
- Price, Nicholas and Dwek, Raymond and Wormald, Mark. Principles and Problems in Physical Chemistry for Biochemists. R. G. Ratcliffe. New York: Oxford University Press, 1997.
- Irwin H. Segel, Biochemical Calculations (How to Solve Mathematical Problems in General Biochemistry), 2nd edition, John Wiley & Sons, 1975
This format can be adapted for various clinical laboratory tests involving spectrophotometry by adjusting the specific details to match the methodology used for each type of assay.
Possible References Used