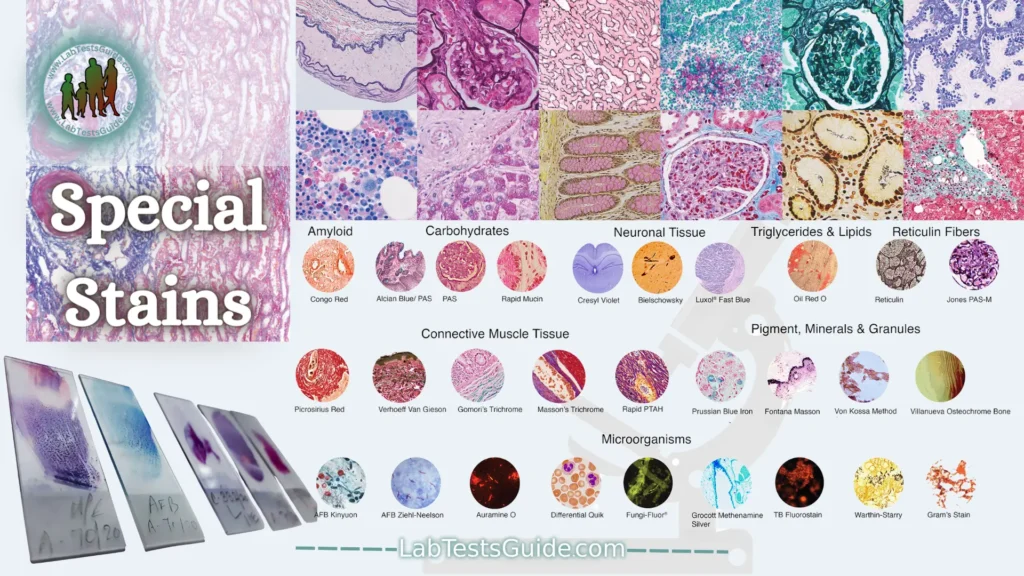
There are a wide variety of special stains to demonstrate pathologic processes. They generally employ a dye or chemical which has an affinity for the particular tissue component to be demonstrated.
(H&E) Hematoxylin and Eosin Stain:
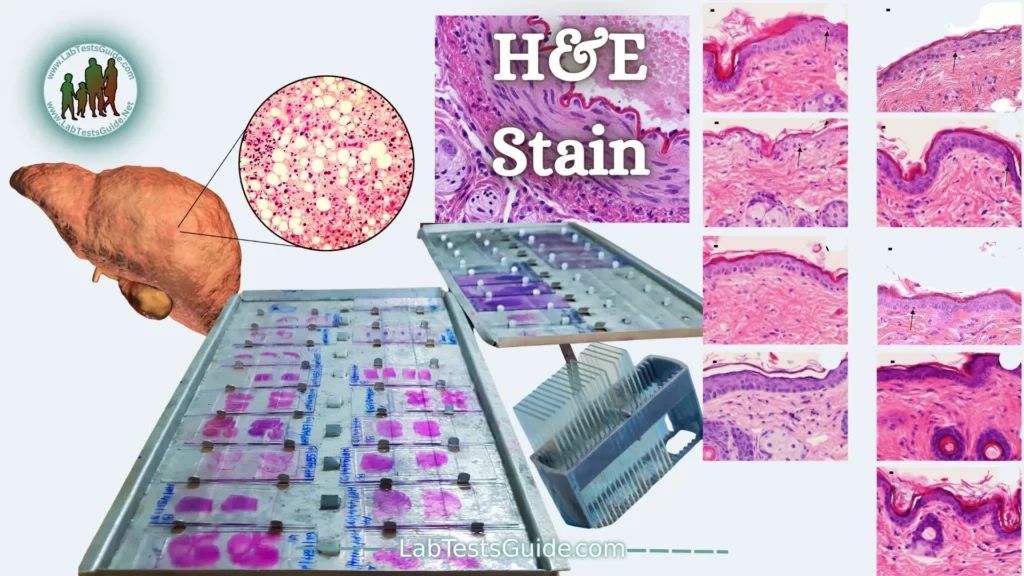
Hematoxylin and Eosin (H & E) staining is routinely used in histopathology laboratories as it allows pathologists and researchers to obtain a very detailed view of the tissue. This is because staining of cell structures such as cytoplasm, nucleus, organelles and extracellular components is performed. This information is sometimes sufficient to establish a diagnosis of the organization (or disorganization) of the cells of the tissue and also shows the abnormalities or the particular indicators of the cells (like the changes of the nucleus typical of certain cancers). Even when other more complex methods of staining are used, H & E staining continues to be part of the diagnosis because it allows one to see the morphology of the tissue which will then allow the pathologist or researcher to interpret the other stains.
In the laboratories, all the samples are initially stained by the H & E method and the specific stains are carried out only if it is necessary to have more information to obtain a more detailed analysis, for example to differentiate 2 types of cancer having a similar morphology.
Eosin:
Eosin is an acid, which has a selective affinity for the cellular cytoplasm (plant or animal); it is preferably attached to the basic molecules and can replace the carmine dye for the preparations. It is often used with hematoxylin, which stains nuclei well.
Eosin is therefore widely used as a dye for laboratory microscopy, to stain the cytoplasm of cells, collagen, muscle fibers, lymphocytes and bacteria.
A class of granulocytes, the eosinophils, whose number increases during allergies and certain parasitoses, derive their name from this dye.
Hematoxylin:
Hematoxylin is a basic dye that gives a blue or blue-black color to acidic substances in cells and tissues. Thus the chromatin of the nuclei composed of desohyribonucleic acid is colored in dark blue by hematoxylin. In the same way, the cytoplasm is colored more or less intensely in blue due to the ribosomes which contain the ribonucleic acid. Substances or structures that stain blue with hematoxylin are called basophilic.
Hematoxylin is very commonly used in histology laboratories for routine staining. It is often associated with eosin to obtain a complete labeling of cellular structures.
Connective Tissue Stains:

Connective tissue (CT) is one of the four types of animal biological tissue that supports, unites or distinguishes different types of tissues and organs in the body. It originates in the mesoderm, at the time of gastrulation, during embryonic development. The other three types are epithelium, muscle tissue, and nervous tissue. Connective tissue finds its place among other tissues in the body, such as those of the nervous system. Connective tissue includes the outer membranes that surround the brain and spinal cord, which establish the central nervous system.
The connective tissue cells are fibroblasts, adipocytes, macrophages, mast cells and leukocytes.
Special connective tissue stains are used to highlight connective fibers. Connective fibers include collagen, reticulin, and elastic fibers. Collagen fibers are the most abundant of the 3. They are found throughout the body except in the central nervous system.
Massons Trichrome Stain:
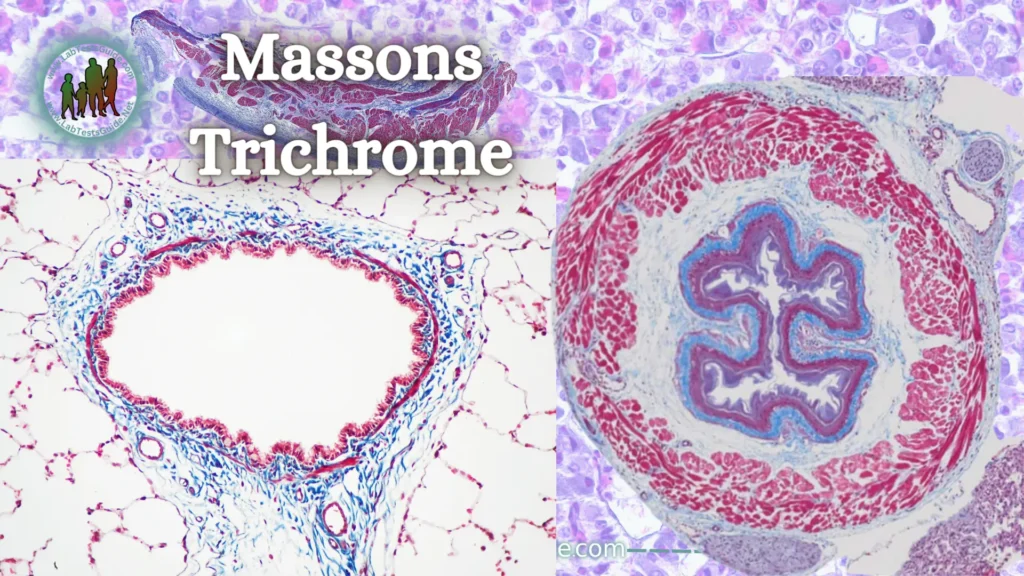
Masson’s trichrome is a dye that highlights collagen fibers. This method is very popular and is used in histology to differentiate collagen and muscle fibers in tissue sections. It is especially used in the study of pathologies of the heart (infarction), liver (cirrhosis), and kidney (glomerular fibrosis).
Most recipes stain keratin and muscle fibers red, collagen and bones blue or green, cytoplasm light red or pink, and nuclei black.
The technique called “Masson’s trichrome” is applied to sections of animal tissues and consists of 3 successive stains:
- Staining of the nuclei with Mayer’s hematoxylin or Harris’ hematoxylin
- Staining of the cytoplasm using a precise mixture of acid fuchsin and sewer red (which are acid dyes).
- Elective collagen staining with light green (sometimes replaced by aniline blue)
Verhoff’s Elastic Stain:
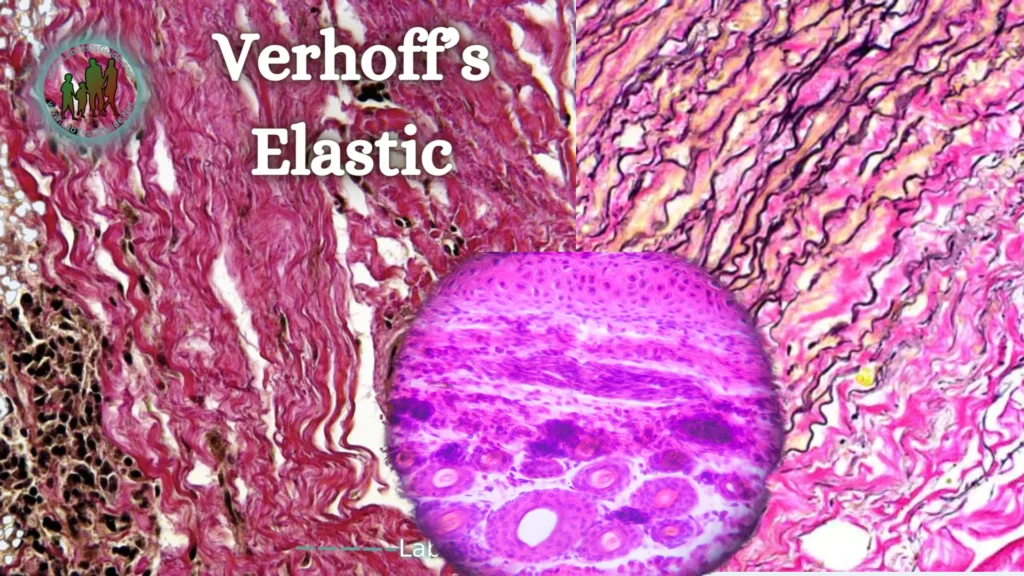
This stain is useful in the diagnosis of congenital or acquired degenerative lesions (streaks, solar elastosis), vasculitis (it may be necessary to demonstrate the elastic limit of the vessels) or temporal arteritis.
This method is based on staining elastic fibers with hematin combined with iodine and ferric chloride. The Verhoeff coloring kit contains 6 dyes.
The method is based on the affinity of hemalun associated with a Lugol’s solution for elastic fibers. The selectivity of the method is not absolute; other tissue structures, such as collagen and muscles, can be stained; Therefore, it is important to perform careful differentiation to obtain a marked and selective coloration of the elastic fibers. Red background staining with Sirius picrate can complement the chromatic framework to highlight other components of the connective tissue.
Verhoeff stain highlights different cellular and tissue structures:
- The nuclei from gray to black.
- Elastic fibers in black.
- Staining of cytoplasm, red blood cells or collagen depends on the counterstain used.
Reticulin Stain:
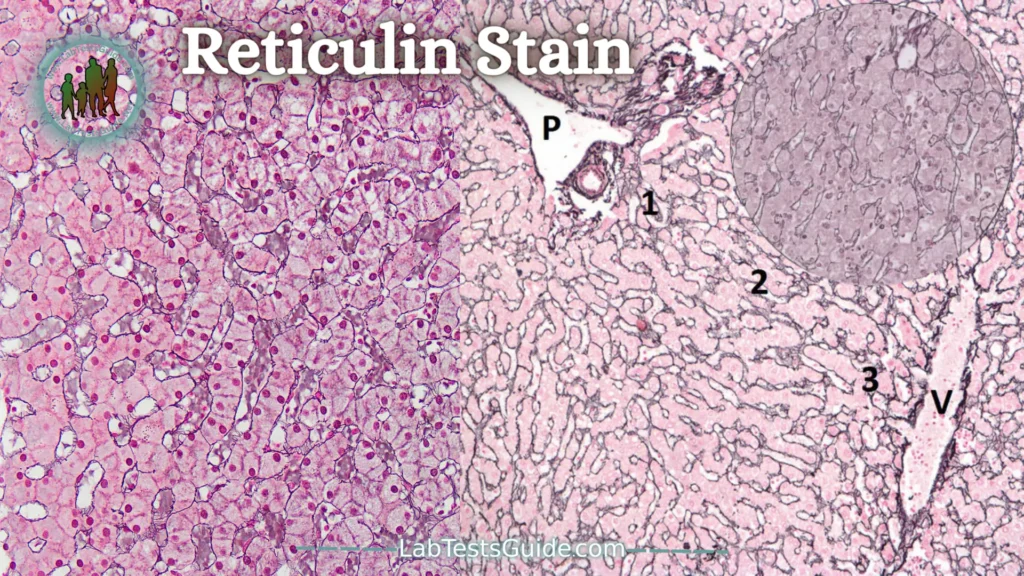
Reticulin staining is a stain that stains the reticulin fibers of the stroma and allows the architecture of tumors to be specified (such as follicular architecture).
It is very useful in cases where the tumor is necrotic. It is a key stain in the study of lesions that affect organs whose structure is rich in reticulin such as the liver (fibrosis, in which this color shows the youngest collagen), the spleen and the lymph nodes (reticulin network that constitutes the skeleton), bone. marrow (myelofibrosis).
Reticulin staining is based on silver impregnation of reticulin fibers that cannot be detected with hematoxylin and eosin staining. In fact, this coloration takes advantage of the argyrophilic properties of reticulin fibers. With this staining, the reticulin fibers are stained black.
The stages of reticulin staining:
- The fibers are first oxidized, usually with potassium permanganate, and then bleached with oxalic acid, potassium metabisulfite, or periodic acid.
- Sensitization is a prepreg through the formation of organometallic complexes between the reticulin fibers and metallic salts, the metallic part being able to be exchanged with silver. The preferred sensitizers are ferric salts.
- For the impregnation stage, an ammoniacal solution of silver nitrate is most often used. Silver will be deposited on the reticulin fibers as a brown oxide which will convert to metallic silver during reduction.
- Ionic silver is reduced to metallic silver in the presence of a developing agent, formalin. We obtain a black deposit visible to the naked eye.
- Turning is a treatment of gold chloride cuts in which silver is replaced with gold to obtain better resolution: a lighter background and a finer, more stable metal deposit.
- An additional reduction is required, fixation with sodium thiosulfate, which removes nonspecific ionic silver or gold residues.
- Counterstaining: it is chosen based on the solubility of the metal that impregnates the fibers. Most often neutral red is used.
Giemsa Stain:

Giemsa dye is based on the use of Giemsa dye, a neutral blue dye composed of an acidic dye, eosin, and a basic dye, methylene azide, which is metachromatic (= having the property of giving certain fabrics a tone different from its own color). An acetic acid bath removes excess blue and reveals the different tissue elements whose color varies depending on the molecules fixed by the dye.
Giemsa staining allows you to demonstrate the presence of microorganisms in all types of tissues. It can be used especially to detect the presence of Helicobacter pylori in the diagnosis of gastric ulcers or chronic gastritis. It can also detect the Leishmaniasis parasite or the fungus Aspergillus niger, which may be responsible for pulmonary mycosis. It also allows visualization of the different blood cells in the hematopoietic tissues.
Giemsa staining is performed on paraffin sections. It is used to stain blood cells in hematopoietic tissues. It can also be applied to all tissue sections in which the presence of microorganisms is suspected. Gram + and Gram bacteria are not differentiated with this stain.
Below are some examples of stains obtained with Giemsa stain:
- Helicobacter pylori: blue, with its characteristic “seagull flight” shape
- Cores: Blue
- Cytoplasm: pink
Microorganisms Stains:
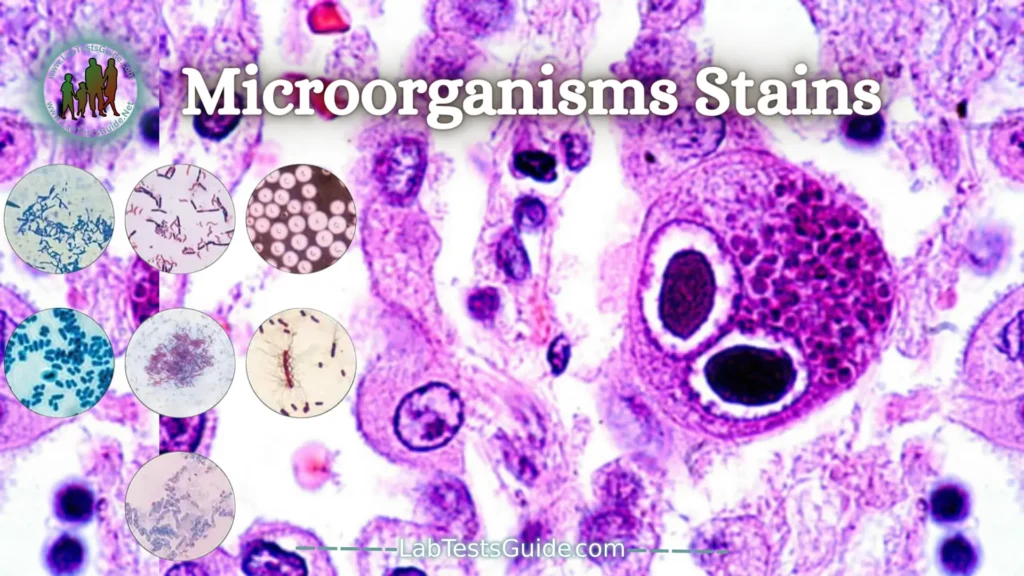
Microorganism stains in histopathology are laboratory techniques designed to improve the visualization of microorganisms, including bacteria, fungi, and parasites, in tissue samples. These stains involve the use of specific dyes or chemicals that selectively interact with microbial structures, aiding in the identification and analysis of microorganisms within histological specimens under the microscope. Examples include the Gram stain for bacteria and the periodic acid Schiff (PAS) stain for fungi in histopathological specimens.
Brown and Hopps Stain:
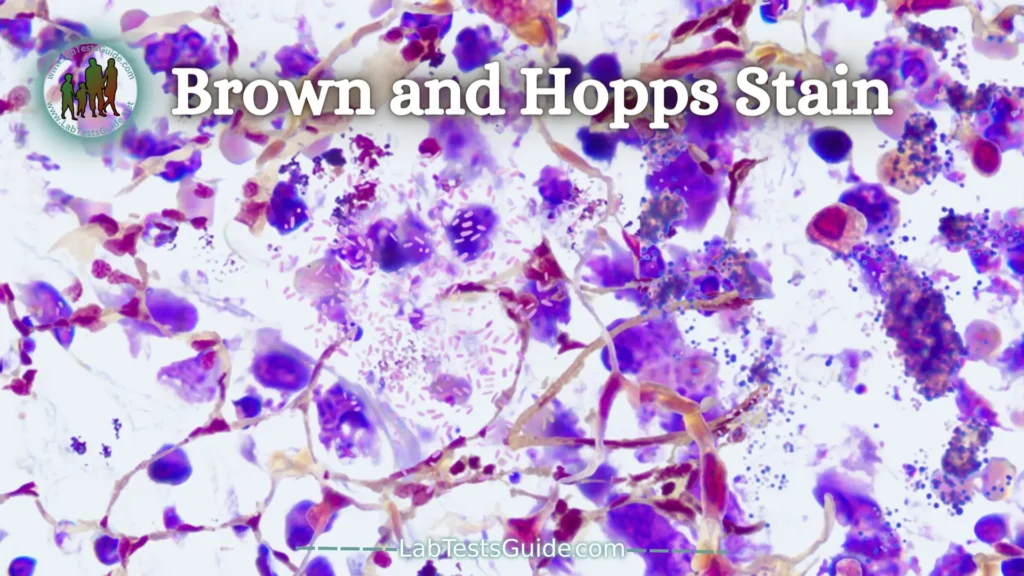
Bacteria appear on H and E as blue rods or cocci regardless of gram reaction. Colonies appear as fuzzy blue clusters. Tissue gram stains are all basically the same as that used in the microbiology lab except that neutral red is used instead of safranin. Gram positive organisms usually stain well, but gram negatives do not (because the lipid of the bacterial walls is removed in tissue processing).
Staining Procedure
- Deparaffinize and hydrate to distilled water.
- Place slides on staining rack and pour on Crystal Violet Solution, 1%, Aqueous for two minutes. Rinse in distilled water.
- Mordant in Gram’s Iodine for five minutes. Rinse in distilled water.
- Differentiate in Cellosolve, until blue color no longer streams away from the section (approximately 5-10 seconds). (See note)
- Quickly rinse in distilled water. (See note)
- Stain in Basic Fuchsin Solution, 0.5%, Aqueous for five minutes. Rinse in distilled water.
- Gallego’s Solution , for five minutes. (Differentiates and “fixes” the Basic Fuchsin).
- Rinse thoroughly in distilled water and blot lightly to remove excess water (not to dryness).
- Stain for three seconds in Tartrazine Solution . Immediately blot away excess, but not to dryness).
- Cellosolve three changes, for six quick dips in each. (See note)
- Xylene, three changes, for 10 dips each. Slides may remain in Xylene until ready for mounting.
- Mount with Permount.
| Gram Negative Bacteria | Red |
| Gram Positive Bacteria | Blue |
| Nuclei | Red |
| Background | Yellow |
Note:
- Preferably, all stains and solutions are applied to the slide while it is in a horizontal position, except for steps 4, 10 and 11, where the slide should be dipped into the solutions contained in coplin jars.
- Steps 4, 5, 9 and 10 are critical with regard to timing.
- This procedure does not work well on Bouin’s fixed tissues.
AFB (acid-fast bacilli) stain:

This stain uses carbol-fuchsin to stain the lipid walls of acid fast organisms such as M. tuberculosis. The most commonly used method is the Ziehl-Neelsen method, though there is also a Kinyoun’s method. A modification of this stain is known as the Fite stain and has a weaker acid for supposedly more delicate M. leprae bacilli. However, much of the lipid in mycobacteria is removed by tissue processing, so this stain can, at times, be very frustrating and cause you to search extensively for organsisms you are sure are in a big granuloma. The most sensitive stain for mycobacteria is the auramine stain which requires a fluorescence microscope for viewing. There are things other than mycobacteria that are acid fast. Included are cryptosporidium, isospora, and the hooklets of cysticerci.
GMS (Gomori Methenamine Silver) Stain:

This stain, often abbreviated as “GMS”, is used to stain for fungi and for Pneumocystis carinii. The cell walls of these organisms are stained, so the organisms are outlined by the brown to black stain. There is a tendency for this stain to produce a lot of artefact from background staining, so it is essential to be sure of the morphology of the organism being sought.
PRINCIPLE: The mucopolysaccharide components of the fungal cell wall
are oxidized to release aldehyde groups. The aldehyde groups then react
with the silver nitrate, reducing it to a metallic silver, rendering them
visible.
Procedure:
Paraffin sections at 5 microns.
All glassware must be chemically cleaned prior to use. Forceps must be Teflon, plastic or paraffin
coated.
- De-paraffin slides in xylene – 2 minutes
- De-paraffin slides in xylene – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Hydrate slides in 95% alcohol – 2 minutes
- Hydrate slides in running water – 5 minutes
- Place in 10% chromic acid – 30 minutes
- Wash in running water – 1 minute
- Rinse in distilled water
- Place in 1% sodium bisulfite – 1 minute
- Rinse in running water – 10 minutes
- Rinse in distilled water – 1 minute
- Place in working Methenamine silver at 60C- 45-60 minutes or until sections turn
yellow brown. Remove the control slide using paraffin coated forceps, rinse in the
warmed distilled water, and microscopically check for adequate silver impregnation.
Fungi should be dark brown. Rinse in distilled water and return to the silver solution if
reaction is too pale. - Rinse in 6 changes of Distilled water
- Place in 0.1% gold chloride – 2-5 minutes
- Solution may be re-filtered and re-used for about 100 slides.
- Rinse in Distilled water – 1 minute
- Place in 2% sodium thiosulfate – 4 minutes
- Rinse in distilled water – 1 minute
- Place in working light green – 2 minutes
- Dehydrate in 95% alcohol – 1 minute
- Dehydrate in 100% alcohol – 1 minute
- Dehydrate in 100% alcohol – 1 minute
- Dehydrate in 100% alcohol 5 minutes
- Clear in xylene – 2 minutes
- Clear in xylene – 5 minutes
- Time: 1 hour 58 minutes
Results:
Fungi – Sharply delineated in Black
Pneumocystis carnii – black
Mucin – Taupe to dark Grey
Inner parts of mycelia and hyphae – Old Rose
Background – Pale Green
PAS (periodic acid-Schiff) Stain:

PAS (Periodic Acid Shiff) stain is the most versatile and widely used technique for carbohydrate visualization.
From a diagnostic point of view, PAS is one of the most useful and used special stains in the pathology laboratory. It can help on the one hand to the differential diagnosis of tumors and on the other hand to detect fungal diseases.
PAS stain showed mucins (brush-edged epithelium), basement membranes, glycogen, and mycelial spores and filaments. The reaction with the periodic acid of Schiff corresponds to the oxidation of certain polysaccharides by the periodic acid, revealed by a red coloration (fixation of the Schiff leucofuchsin). PAS is commonly associated with diastase (PAS-diastase) which digests glycogen but leaves the pink stain of intra- or extracellular mucins persistent. In undifferentiated tumors, this staining is useful for guiding the diagnosis to a glandular origin (adenocarcinoma). It is required in the diagnosis of pathologies of the liver and the kidney.
Carbohydrate Stains:
Colloidal iron (“AMP”) Stain:
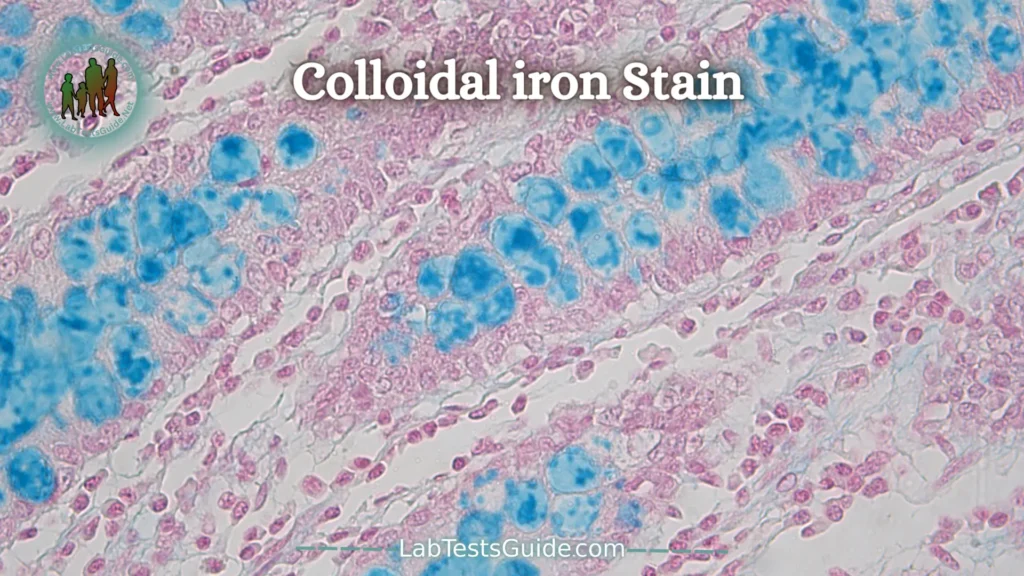
Iron particles are stabilized in ammonia and glycerin and are attracted to acid mucopolysaccharides. It requires a formalin fixation. Phospholipids and free nucleic acids may also stain. The actual blue color comes from a Prussian blue reaction. Tissue can be pre-digested with hyaluronidase to provide more specificity.
Colloidal Iron (AMP) techniques will also demonstrate mucin but require formalin fixation. The pH is critical and can be adjusted to give more specificity. The blue color is demonstrated by a Prussian Blue reaction.
Positive Control – Human Small intestine
Fixation – 10% Neutral Buffered Formalin
Section thickness 5 – 10 microns
Protocole:
- De-paraffin slides in xylene – 2 minutes
- De-paraffin slides in xylene – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Hydrate slides in 95% alcohol – 2 minutes
- Hydrate slides in running water – 5 minutes
- Rinse slides in 12% acetic acid
- Working colloidal iron solution – 60 minutes
- Rinse slides in 12% acetic acid, 3 changes, 3 minutes each
- Ferrocyanide-hydrochloric acid solution – 20 minutes
- Wash in running tap water – 5 minutes
- Counterstain with nuclear fast red – 5 minutes
- Wash in tap water – 3 minutes
- Dehydrate in 95% alcohol – 30 seconds
- Dehydrate in 100% alcohol – 1 minute
- Dehydrate in 100% alcohol – 5 minutes
- Clear in xylene – 2 minutes
- Clear in xylene – 5 minutes
- Coverslip
Results:
Acid mucopolysaccharides and sialomucins – Deep blue
Nuclei –Pink-red
Cytoplasm – Pink
Alcian Blue Stain:
The alcian blue stain is used to stain acidic mucus and acetic mucins.
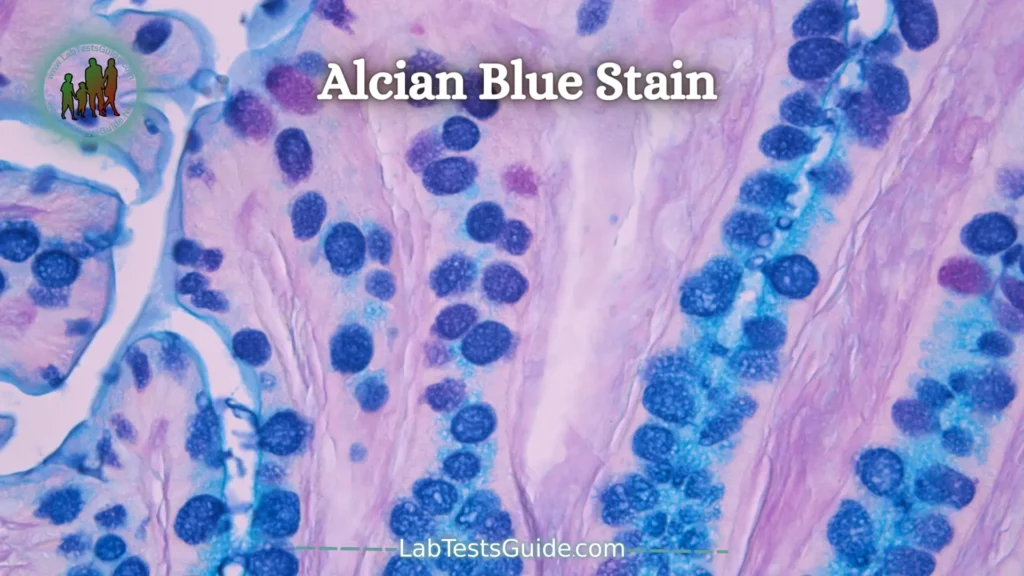
Excessive amounts of non-sulfated acidic mucus are observed in mesothelioma. These phlegm are present in normal amounts in the walls of the blood vessels but increase in early lesions of atherosclerosis.
Alcian blue staining is based on a group of versatile basic dyes that are soluble in water. The blue color is due to the present copper.
The pH of the Alcian blue staining solution is very important and has a direct effect on the category of mucins revealed by this method. The pH of the Alcian blue staining solution can be between 0.4 and 2.5.
If the pH of the solution is 1.0, the solution will stain the sulfated acid mucins. These mucins are mainly found in the cartilage, the goblet cells of the large intestine and the bronchial serous
If the Alcian blue staining solution has a pH of 2.5, it is the carboxylated mucins in the connective tissues and the cartilage that will be stained.
- De-paraffin slides to water
- 3% acetic acid – 3 minutes
- Alcian blue 2.5 solution – 30 minutes
- Wash in running water – 10 minutes
- 0.1% nuclear fast red – 5 minutes
- Rinse in running water – 30 seconds
- Dehydrate in 95% alcohol – 30 seconds
- Dehydrate in 100% alcohol – 1 minute
- Dehydrate in 100% alcohol – 2 minutes
- Dehydrate in 100% alcohol – 2 minutes
- Clear in xylene – 2 minutes
- Clear in xylene – 5 minutes
Results:
Weakly acidic sulfated Mucosubstances,
hyaluronic acid and sialomucins- Dark blue
Background- Pink to Red
PAS (peroidic acid-Schiff) Stain:
PAS (Periodic Acid Shiff) stain is the most versatile and widely used technique for carbohydrate visualization.

From a diagnostic point of view, PAS is one of the most useful and used special stains in the pathology laboratory. It can help on the one hand to the differential diagnosis of tumors and on the other hand to detect fungal diseases.
PAS stain showed mucins (brush-edged epithelium), basement membranes, glycogen, and mycelial spores and filaments. The reaction with the periodic acid of Schiff corresponds to the oxidation of certain polysaccharides by the periodic acid, revealed by a red coloration (fixation of the Schiff leucofuchsin). PAS is commonly associated with diastase (PAS-diastase) which digests glycogen but leaves the pink stain of intra- or extracellular mucins persistent. In undifferentiated tumors, this staining is useful for guiding the diagnosis to a glandular origin (adenocarcinoma). It is required in the diagnosis of pathologies of the liver and the kidney.
Mucicarmine Stain:
Mucicarmine stain is intended for the staining of mucin. Mucin is a secretion produced by a variety of epithelial cells and connective tissue cells. In certain intestinal inflammations or carcinomas, an excess of mucin is secreted by the epithelial cells.

The mucicarmine staining technique is also useful for determining the site of a primary tumor by visualizing tumor cells producing mucin in an area not containing mucin-producing cells, which means that the tumor is not affected. appeared in this area.
Mucicarmine staining is also useful for staining encapsulated fungi like cryptococcus.
The principle of mucicarmine staining is based on the presence of aluminum that forms a chelating complex with carmine. This changes the charge of the carmine molecule to a positive charge which allows it to bind to low density acidic substrates such as mucins.
Safety equipment: Work under a hood with lab coat, gloves, and glasses.
- De-paraffin slides in xylene – 2 minutes
- De-paraffin slides in xylene – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Hydrate slides in 95% alcohol – 2 minutes
- Hydrate slides in running water – 5 minutes
- Place in Weigerts – 7 minutes
- Wash in running water – 10 minutes
- Place in diluted mucicarmine – 60 minutes
- Rinse quickly in distilled water
- Metanil Yellow – 1 minute
- Rinse quickly in distilled water
- Dehydrate quickly
- Clear in xylene – 2 minutes
- Clear in xylene – 5 minutes
Results:
Mucin – Deep rose to red
Capsule of Cryptococcus – Deep rose to red
Nuclei – Black
Other tissue elements – Yellow
Pigments, Minerals and Cytoplasmic Granules Stains:
Fontana-Masson for Melanin Stain:
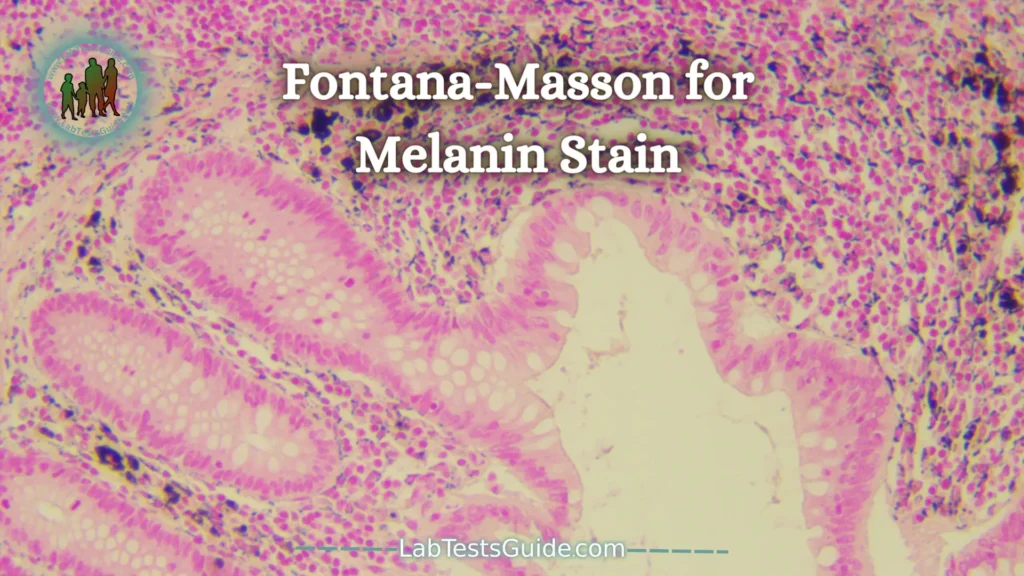
Masson – Fontana stain is based on the properties of melanins. Melanins are a group of brown-black precipitates in the exact chemical structure is not known.
Melanins bind to proteins and melanin-protein complexes are localized in the cytoplasm of cells in melanin granules. Melanin has the ability to reduce ammonia silver nitrate solutions to metallic silver without the use of external reducing agent. Masson – Fontana stain is far from being specific for melanin, because it also makes it possible to visualize other substances which have the same properties of reduction, as the argyrophilic granules and the lipofuschines.
Masson – Fontana stain can be used for the differentiation of brown pigments (lipofuschine, hemosiderin, melanin), for the deposition of pigment due to treatments like Minocycline type II or for the differential diagnosis of hypomelanosis.
Safety equipment: Work under a hood with lab coat, gloves, and glasses.
- De-paraffin slides in xylene – 2 minutes
- De-paraffin slides in xylene – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Clear slides in 100% alcohol – 2 minutes
- Hydrate slides in 95% alcohol – 2 minutes
- Hydrate slides distilled water – 5 minutes
- Immerse slides in silver nitrate at 56C – 60 minutes
- Check slides, granules should be dark brown. Return to solution if necessary.
- Rinse in distilled water – 1 minute
- 0.2% gold chloride – 10 minutes
- Rinse in distilled water – 1 minute
- 5% sodium thiosulfate – 5 minutes
- Rinse in distilled water – 1 minute
- Nuclear fast red – 5 minutes
- Wash in running water – 2 minutes
- Dehydrate in 95% alcohol – 1 minute
- Dehydrate in 100% alcohol – 1 minute
- Dehydrate in 100% alcohol – 2 minutes
- Clear in xylene – 2 minutes
- Clear in xylene – 5 minutes
- Melanin: black
- Aryophilic cellular granules: black
- Certainrs lipofuschines: black
- Cores: Red
- Nuclei – Pink
Melanin Bleach Stain:

Bleaching techniques remove melanin in order to get a good look at cellular morphology. They make use of a strong oxidizing agent such as potassium permanganate or hydrogen peroxide. Ocular melanin takes hours to bleach, while that from skin takes minutes.
PROCEDURE:
- Deparaffinize and hydrate to distilled water.
- Slide for bleach: potassium permanganate solution, 2-4 hours.
- Slide for control, leave in distilled water until step 6.
- 1% Oxalic acid, 1-2 minutes, or until colorless.
- Wash in tap water.
- H & E stain both slides simultaneously.
- Dehydrate, clear, and coverslip.
RESULTS:
If the pigment is melanin it will not be present on the slide that was
bleached.
Iron Stain:
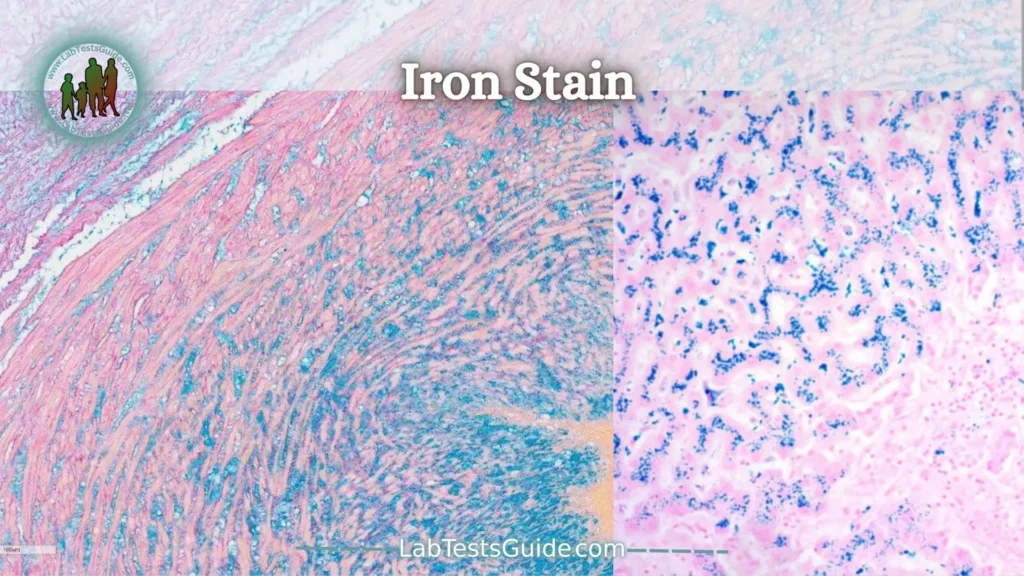
Iron staining of bone marrow aspirate smears is usually part of the standard ordering protocol for bone marrow aspirates. The iron staining procedure uses Prussian blue staining for ferric iron to assess iron stores in the bone marrow. This procedure is particularly useful when evaluating patients with anemia, iron overload, myelodysplasia, etc. In the adult setting, it is commonly performed on bone marrow biopsy, but can also be requested on aspirates.
In the pediatric setting, it is less likely to be part of the standard order, as young children rarely have stainable iron stores. However, iron staining may be ordered in patients with congenital anemia and possible mitochondrial defects to look for sideroblastic anemia.
In this technique, iron is stained blue and is normally found in the bone marrow stroma/macrophages, which are found in the spicules. In aspirated smears, without fragments/spicules, it is not possible to evaluate iron deposits. However, if nucleated red blood cells (NRBCs) are present, it is still possible to look for ringed sideroblasts, common in sideroblastic anemias.
The image on the right is a field of a bone marrow slide from a patient with congenital sideroblastic anemia. The NRBC indicated by the red arrow is a normal siderocyte with few hemosiderin granules scattered throughout the cytoplasm. The NRBC that is indicated by the blue arrow has a large number of hemosiderin granules concentrated in the mitochondria surrounding the nucleus. This is a pathological ringed sideroblast.
VonKossa Stain:
The von Kossa stain is widely used in histology to detect the presence of abnormal calcium deposits in the body. The principle of this coloration is based on the transformation of calcium salts into silver salts: calcium ions, bound to phosphates, are replaced by silver ions provided by a silver nitrate solution. Placed under a light source, silver phosphates undergo photochemical degradation, leading to the observation of metallic silver deposits. Counterstaining with nuclear red allows the nuclei of the cells to be stained red and their cytoplasm pink.
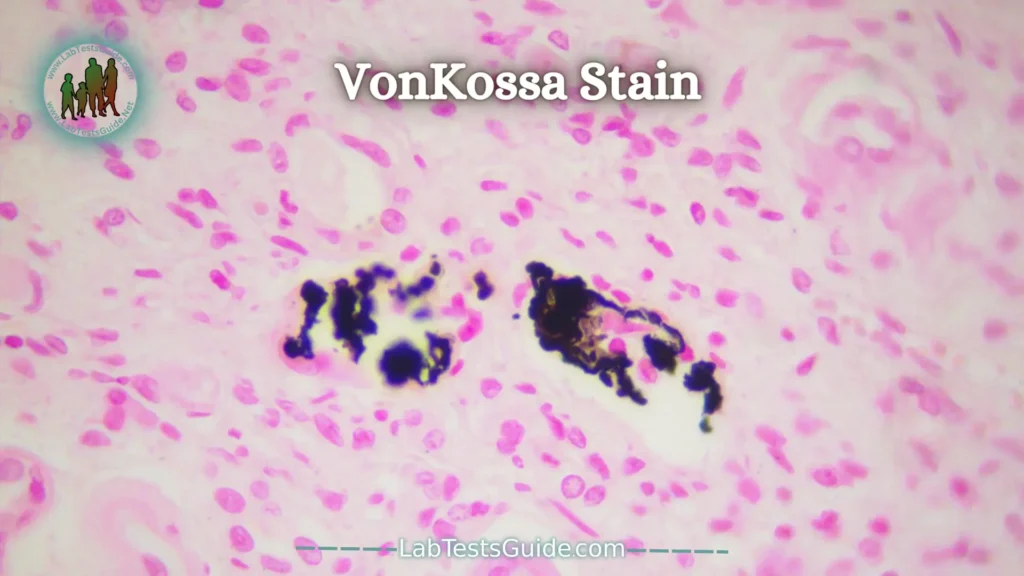
This stain is useful for the diagnosis of calcinosis (e.g. chondrocalcinosis, nephrocalcinosis, cutaneous calcinosis…). It can also be used to detect calcifications in tumors.
The von Kossa stain can be used on all types of tissue that may have abnormal calcium deposits. The joints, blood vessels and kidneys are the most affected.
Safety equipment: Work under a hood with lab coat, gloves, and glasses.
- De-paraffin slides in xylene (1) —————– 2 minutes
- De-paraffin slides in xylene (2) —————— 2 minutes
- Clear slides in 100% alcohol——————— 2 minutes
- Clear slides in 100% alcohol——————— 2 minutes
- Hydrate slides in 95% alcohol——————- 2 minutes
- Hydrate slides in running water—————– 5 minutes
- Place in silver nitrate for 60 minutes exposed to direct sunlight, ultra-violet lamp, or a 100-watt lamp light.
- Rinse in DH2O ————————————– 1 minute
- Place in 5% sodium thiosulfate —————– 2 minutes
- Rinse in DH2O ————————————– 2 minutes
- Place in nuclear fast red ————————– 5 minutes
- Rinse in DH2O ————————————– 30 seconds
- Dehydrate in 95% alcohol———————— 30 seconds
- Dehydrate in 100% alcohol———————- 1 minute
- Dehydrate in 100% alcohol———————- 2 minutes
- Dehydrate in 100% alcohol———————- 5 minutes
- Clear in xylene (3) ———————————- 2 minutes
- Clear in xylene (4) ———————————- 5 minutes
- Cover slip
Results:
Calcium salts —————————– Black
Nuclei ————————————— Red
Cytoplasm ——————————— Light pink
Fat Stains:
Fat stains are laboratory techniques used in histology and pathology to visualize and characterize the presence of fat or lipid deposits within tissues. These stains help researchers and pathologists identify and evaluate the distribution of fat in various organs and structures, providing valuable information for the diagnosis and study of conditions related to lipid metabolism or storage. Examples of lipid stains include Oil Red O and Sudan Black, which selectively bind to lipid molecules and allow them to be visualized under the microscope.
Oil Red O (ORO) Stain:
Oil Red O staining is based on the use of a lysochrome (fat-soluble dye) and an azo dye used for the visualization of triglycerides and neutral lipids in tissue sections.
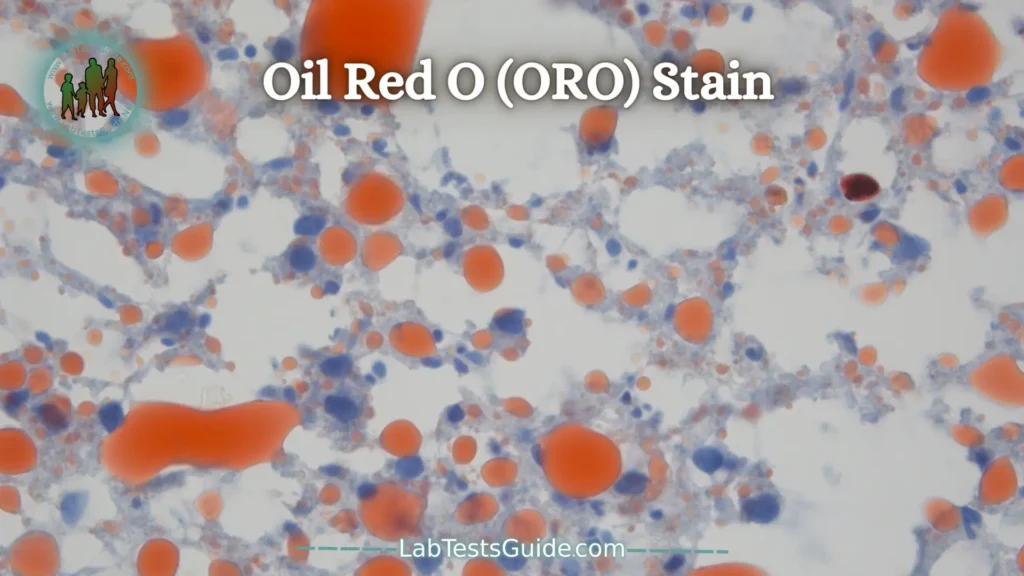
Oil Red O is one of the Sudan dyes such as Sudan III, Sudan IV and Sudan Black B. However, Oil Red O has gradually replaced Sudan III and Sudan IV dyes because the red coloration produced by Oil Red O is much more intense. which makes viewing easier.
Oil Red O staining is performed on fresh or frozen samples. Paraffin-embedded tissues are not compatible with this staining because section preparation and especially the use of solvents remove lipids.
Oil Red O staining is based on the use of 4 reagents for staining fat cells according to the Johnson method.
Safety equipment: Work under a hood with lab coat, gloves, and glasses.
Use on frozen sections only
- Hydrate slides in running water——————— 10 minutes
- Stain in working Oil Red O ————————– 10 minutes
- Rinse in running water ——————————- 2 minutes
- Rinse in 60% isopropanol ————————— 5 minutes
- Rinse in running water ——————————- 2 minutes
- Counterstain with Harris Hematoxylin ———– 20 seconds
- Blue in running water ——————————– 5 minutes
- Coverslip with glycerin jelly or aqueous mounting media
Results:
Fat Globules – Orange Red
Nuclei – Blue
Sudan Black B Stain:
Sudan Black B stain is intended for visualization of lipids. Black Sudan B is used for staining a wide variety of lipids such as phospholipids, sterols and neutral triglycerides. Black Sudan B is not lipid specific like other Sudan dyes and can also be used for staining chromosomes, Golgi apparatus and leukocyte granules.
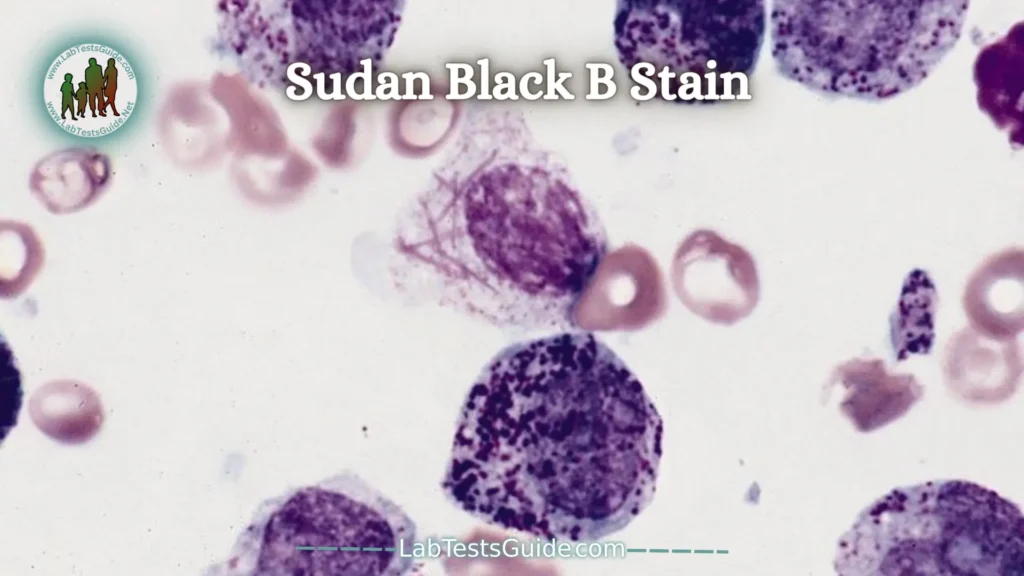
Sudan family dyes form a group of solvent-soluble lipid dyes. They are called lysochromes and in the structural classification they are diazo dyes.
Black Sudan B is used in the study of hematological pathologies because it is capable of staining myeloblasts and not lymphoblasts.
Normal granulocyte precursors show increased sweating that approximately corresponds to the number of granules. Promyelocytes contain some sudanophilic granules, and mature neutrophils contain a large number of sudanophilic granules. The eosinophilic granules are also sudanophilic, especially at the edge. Monocytes may be colorless or contain a few discrete sudanophilic granules. Macrophages usually contain sudanophilic material.
Possible References Used