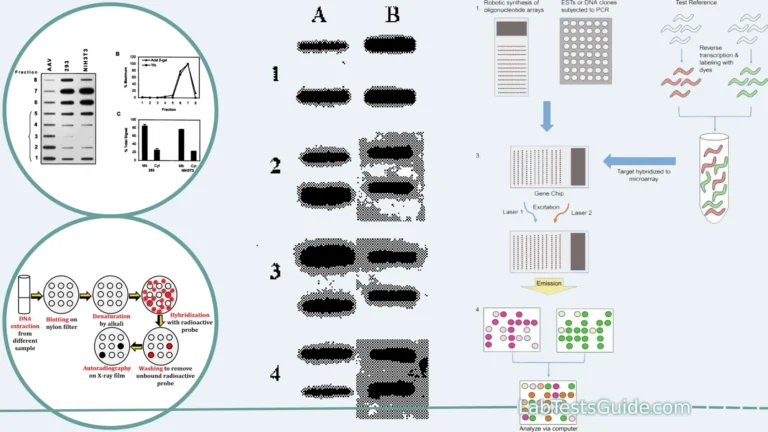
Reverse Transcription PCR (RT-PCR) is a technique used to detect and quantify RNA expression by converting RNA into complementary DNA (cDNA) using reverse transcriptase, followed by amplification using PCR. It is widely used for diagnosing viral infections, gene expression analysis, and detecting RNA biomarkers.

Methodology for Reverse Transcription PCR (RT-PCR) in Clinical Laboratory
Introduction
Reverse Transcription PCR (RT-PCR) is a technique used to detect and quantify RNA expression by converting RNA into complementary DNA (cDNA) using reverse transcriptase, followed by amplification using PCR. It is widely used for diagnosing viral infections, gene expression analysis, and detecting RNA biomarkers.
Principle
RT-PCR involves two main steps: reverse transcription (RT) of RNA to cDNA, and polymerase chain reaction (PCR) amplification of specific cDNA sequences. The process is monitored in real-time (real-time RT-PCR) using fluorescent probes or dyes to quantify the amount of target RNA.
Specimen Requirements
- Type of specimen: Blood, plasma, serum, tissue samples, swabs, or other biological fluids
- Volume of specimen required: Typically 200 µL to 1 mL
- Collection method: Standard clinical procedures for sample collection (e.g., venipuncture, swabs)
- Handling and storage requirements: Store samples at -80°C for long-term storage; RNA is highly labile, so avoid repeated freeze-thaw cycles
- Stability of the specimen: RNA is prone to degradation; process samples promptly or stabilize RNA using commercially available RNA stabilization reagents
Reagents and Materials
- RNA extraction kit
- Reverse transcriptase enzyme and reaction buffer
- Random primers or oligo(dT) primers
- dNTP mix
- RNase inhibitor
- PCR master mix (including DNA polymerase, buffer, dNTPs, MgCl2)
- Specific primers and probes for target RNA
- Nuclease-free water
- Positive and negative control RNA
Equipment
- Thermal cycler or real-time PCR machine
- Microcentrifuge
- Vortex mixer
- Micropipettes and RNase-free tips
- RNase-free tubes and consumables
- Biosafety cabinet (for handling RNA)
Procedure
RNA Extraction:
- Extract RNA from the sample using an RNA extraction kit following the manufacturer’s instructions.
- Quantify the extracted RNA using a spectrophotometer or fluorometer.
- Assess RNA integrity using agarose gel electrophoresis or a bioanalyzer (optional).
Reverse Transcription:
- Prepare the reverse transcription reaction mix:
- RNA template: 1-2 µg
- Reverse transcription buffer: 1X
- dNTP mix: 1 mM each
- Reverse transcriptase enzyme: as per manufacturer’s instructions
- Primers (random primers or oligo(dT)): 10-20 µM
- RNase inhibitor: as per manufacturer’s instructions
- Incubate the reaction mix at 25°C for 10 minutes (if using random primers), followed by 42-50°C for 30-60 minutes to synthesize cDNA.
- Inactivate the reverse transcriptase by heating at 70°C for 10 minutes.
PCR Amplification:
- Prepare the PCR reaction mix:
- cDNA template: 1-2 µL
- PCR master mix: 1X
- Forward and reverse primers: 0.2-0.5 µM each
- Probe (if using): 0.1-0.2 µM
- Nuclease-free water to the final volume (usually 20-25 µL)
- Set up the PCR cycling conditions:
- Initial denaturation: 95°C for 2-3 minutes
- Amplification (40 cycles):
- Denaturation: 95°C for 15-30 seconds
- Annealing: 55-60°C for 30 seconds
- Extension: 72°C for 30-60 seconds
- Final extension: 72°C for 5-10 minutes (if required)
- Analyze the amplification data using the real-time PCR machine software to determine the cycle threshold (Ct) values.
Quality Control
- Include no-template controls (NTC) to check for contamination.
- Use positive and negative controls to validate the reverse transcription and PCR efficiency.
- Monitor the amplification efficiency and specificity by analyzing the melting curves or gel electrophoresis of the PCR products.
Calculation and Interpretation
- Determine the relative expression levels of the target RNA by comparing the Ct values of the samples to those of the controls or standard curve.
- Normalize the expression levels to a housekeeping gene for accurate quantification.
- Interpret the results within the clinical context, considering potential sources of error such as sample quality, RNA integrity, and assay efficiency.
Limitations
- RNA is prone to degradation, requiring careful handling and storage.
- RT-PCR is sensitive to contamination; rigorous controls and clean working environments are essential.
- Quantification accuracy depends on the efficiency of the reverse transcription and PCR steps.
Safety Precautions
- Use PPE (gloves, lab coat, safety goggles) when handling RNA and reagents.
- Work in an RNase-free environment and use RNase-free reagents and consumables.
- Dispose of waste according to local regulations and institutional guidelines.
References
- Sambrook, J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual (3rd ed.). Cold Spring Harbor Laboratory Press.
- Clinical Chemistry: Principles, Techniques, and Correlations by Michael L. Bishop, Edward P. Fody, and Larry E. Schoeff.
- Manufacturer’s instructions for RNA extraction kits, reverse transcription reagents, and PCR master mixes.
- Clinical Laboratory Standards Institute (CLSI) guidelines for molecular diagnostic methods.
This format provides a detailed methodology for conducting RT-PCR in clinical laboratories, focusing on accurate detection and quantification of RNA expression.
Possible References Used