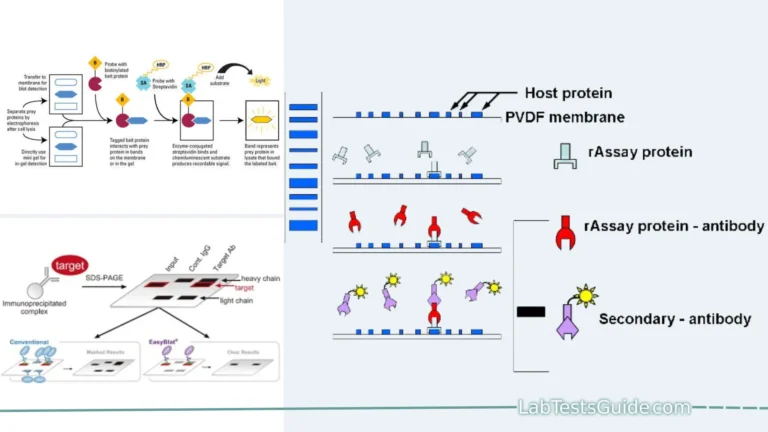
Reverse blotting is a molecular biology technique used to transfer biomolecules such as DNA, RNA, or proteins from a gel to a solid membrane, typically a nitrocellulose or PVDF (polyvinylidene difluoride) membrane. This technique is often employed in various laboratory applications, including Southern blotting, Northern blotting, and Western blotting, to analyze and detect specific target molecules.

Key Points of Reverse Blotting:
- Definition: Reverse blotting is the process of transferring biomolecules from a gel (such as agarose or polyacrylamide gel) to a solid membrane.
- Purpose: It is used to immobilize and preserve separated biomolecules on a membrane for subsequent analysis, such as probing for specific targets.
- Membrane Types: Commonly used membranes are nitrocellulose and PVDF (polyvinylidene difluoride).
- Electrophoresis: Before reverse blotting, biomolecules are separated using gel electrophoresis based on size or charge.
- Staining: After electrophoresis, the gel is stained to visualize the separated biomolecules.
- Transfer Direction: In reverse blotting, transfer occurs from the gel to the membrane, opposite to traditional blotting techniques.
- Electric Field: An electric field is applied to facilitate the transfer of biomolecules from the gel to the membrane.
- Contact Transfer: The gel is placed in direct contact with the membrane during the transfer process.
- Transfer Buffer: A transfer buffer is used to maintain the appropriate ionic strength and pH for efficient transfer.
- Wicking: The membrane wicks or pulls the biomolecules out of the gel onto its surface.
- Fixation: After transfer, biomolecules are fixed to the membrane, often using UV cross-linking or heat.
- Cross-Linking: Cross-linking ensures that the biomolecules remain immobilized on the membrane.
- Types of Blotting: Depending on the type of biomolecules, it can be called Southern blotting (for DNA), Northern blotting (for RNA), or Western blotting (for proteins).
- Probe Binding: After fixation, the membrane is probed with specific labeled DNA, RNA, or antibodies to detect target molecules.
- Signal Detection: Probes bind to complementary sequences or target proteins, and the signal is detected using methods like autoradiography, chemiluminescence, or fluorescence.
- Molecular Weight Marker: A molecular weight marker is often included in the gel for reference during transfer and subsequent analysis.
- Transfer Efficiency: The efficiency of transfer depends on factors like buffer composition, transfer time, and voltage.
- Minimizing Artifacts: Care must be taken to avoid artifacts, such as incomplete transfer or smearing of bands.
- Transfer Apparatus: Specialized transfer apparatus, such as a semi-dry or wet transfer system, may be used for efficient transfer.
- Hybridization: In DNA and RNA blotting, hybridization with labeled probes helps identify specific sequences.
- Applications: Reverse blotting is widely used in molecular biology research, clinical diagnostics, and other fields for analyzing nucleic acids and proteins.
Defination of Reverse Blotting:
Reverse blotting is a laboratory technique used to transfer biomolecules, such as DNA, RNA, or proteins, from a gel onto a solid membrane for further analysis and detection of specific target molecules.
Background and Significance:
History of Reverse Blotting:
- 1970s: Southern blotting, the first nucleic acid blotting technique, is developed by Edwin Southern.
- 1980s: Reverse blotting is developed as a variation of Southern blotting.
- 1990s: Reverse blotting is used to develop a variety of diagnostic tests for genetic diseases and infectious diseases.
- 2000s: Reverse blotting is used in high-throughput gene expression analysis studies.
- Present: Reverse blotting is a widely used technique for a variety of research and diagnostic applications.
Background of Reverse Blotting:
- Background: Reverse blotting was developed as a reverse application of traditional blotting techniques, which involved transferring molecules from membranes to gels.
- Significance: It allows researchers to preserve and analyze biomolecules separated by gel electrophoresis, aiding in various molecular biology experiments.
Significance of Reverse Blotting:
- Background: Reverse blotting is significant in molecular biology, where it plays a crucial role in techniques like Southern, Northern, and Western blotting.
- Significance: It enables the study of specific DNA, RNA, or protein targets, contributing to research in genetics, gene expression, and protein analysis.
Purpose of Reverse Blotting:
- Immobilization on a Membrane: To transfer biomolecules from a gel to a solid membrane, allowing for their preservation and further analysis.
- Target Detection: To facilitate the detection of specific DNA, RNA, or protein targets within the immobilized biomolecules.
- Molecular Analysis: To enable the study of biomolecule properties, such as size, quantity, or post-translational modifications.
- Hybridization Studies: In DNA and RNA blotting, it helps identify complementary sequences or specific genes of interest.
- Quantification: To measure the abundance of specific biomolecules, aiding in quantitative analysis.
- Comparative Analysis: To compare biomolecule profiles across different samples or conditions, revealing differences or similarities.
- Clinical Diagnostics: Used in medical laboratories to detect disease markers or genetic mutations in patient samples.
- Research in Molecular Biology: To advance our understanding of gene expression, protein function, and molecular mechanisms in biological systems.
- Quality Control: In biotechnology and pharmaceutical industries, it ensures the quality and consistency of biomolecule production.
- Forensic Science: To analyze DNA samples for criminal investigations or paternity testing.
Applications of Reverse Blotting:
- Northern Blotting: Employed to study gene expression by detecting and quantifying specific RNA molecules, including mRNA.
- Western Blotting: Used to detect and analyze proteins, including their size, abundance, and post-translational modifications.
- Dot Blotting: Enables the rapid screening and detection of specific biomolecules, often used for antibody screening.
- Slot Blotting: Similar to dot blotting, it is used for efficient analysis and quantification of nucleic acids or proteins.
- Hybridization Studies: Facilitates the identification of DNA or RNA sequences of interest through probe hybridization.
- Clinical Diagnostics: Used in medical laboratories to detect disease markers, such as viral DNA or specific proteins, in patient samples.
- Comparative Analysis: Helps researchers compare biomolecule profiles across different experimental conditions or samples.
- Quality Control: In biotechnology and pharmaceutical industries, it ensures the quality and consistency of biomolecule production.
- Forensic Analysis: Utilized in forensic science for DNA analysis in criminal investigations, paternity testing, and identification.
- Research in Molecular Biology: Essential for studying gene regulation, protein function, and molecular mechanisms in biological research.
- Environmental Monitoring: Used to detect specific microbial or genetic markers in environmental samples, aiding in pollution and biodiversity studies.
Principles of Reverse Blotting:
- Gel Electrophoresis: Before reverse blotting, biomolecules are separated in a gel matrix through gel electrophoresis. The gel may be made of agarose or polyacrylamide, depending on the type of biomolecule being analyzed.
- Staining: After electrophoresis, the gel is often stained with a dye or a radioactive label to visualize the separated biomolecules. This staining helps researchers identify and locate the molecules of interest.
- Transfer Direction: In reverse blotting, the transfer occurs in the opposite direction compared to traditional blotting techniques. Instead of transferring molecules from a membrane to a gel, biomolecules are transferred from the gel to a solid membrane.
- Solid Membrane: A solid membrane, typically made of nitrocellulose or PVDF (polyvinylidene difluoride), is used to capture and immobilize the biomolecules. These membranes have a high binding capacity for DNA, RNA, or proteins.
- Electric Field: An electric field is applied during the transfer process to facilitate the migration of biomolecules from the gel to the membrane. This is typically achieved through the use of a transfer apparatus.
- Transfer Buffer: A transfer buffer is used to maintain the appropriate pH and ionic strength during the transfer. The buffer composition can vary depending on the type of biomolecule being transferred.
- Wicking Action: The membrane has a wicking action that draws the biomolecules out of the gel and onto its surface. This action helps ensure efficient transfer.
- Fixation: After transfer, biomolecules are often fixed or immobilized on the membrane to prevent them from washing off or diffusing away. Common fixation methods include UV cross-linking or heat treatment.
- Target Detection: The immobilized biomolecules on the membrane can be probed with specific DNA, RNA, or protein probes. These probes are typically labeled with radioactive, fluorescent, or chemiluminescent markers.
- Signal Detection: Probes bind to complementary sequences or target proteins within the immobilized biomolecules. The resulting signal is detected using techniques such as autoradiography, chemiluminescence, or fluorescence.
- Analysis: Once the target molecules are detected, they can be analyzed for various purposes, such as quantification, size determination, or identifying specific sequences or proteins of interest.
- Applications: Reverse blotting is used in various applications, including Southern blotting (for DNA), Northern blotting (for RNA), and Western blotting (for proteins), as well as other molecular biology and biochemical assays.
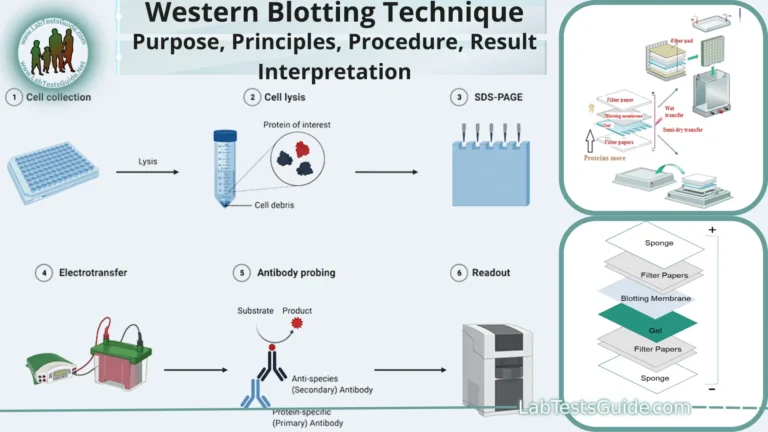
Procedure for Reverse Blotting:
- Gel Electrophoresis: Start with gel electrophoresis to separate biomolecules (DNA, RNA, or proteins) based on size or charge.
- Staining and Visualization: Stain the gel with a dye or radioactive label to visualize the separated biomolecules. Wash off excess stain.
- Prepare Transfer System: Set up the reverse blotting apparatus, including a transfer buffer tank and a solid membrane (nitrocellulose or PVDF).
- Assemble Gel-Membrane Sandwich: Place the gel directly onto the membrane, ensuring there are no air bubbles between them.
- Apply Electric Field: Apply an electric field by connecting the gel and the membrane to the transfer apparatus. This facilitates the transfer of biomolecules onto the membrane.
- Transfer Buffer: The transfer buffer maintains the appropriate pH and ionic strength for efficient transfer. It circulates between the gel and membrane during transfer.
- Fixation: After transfer, fix the biomolecules on the membrane, typically by UV cross-linking or heat treatment.
- Probe Incubation: Incubate the membrane with specific labeled DNA, RNA, or antibodies that will bind to the target biomolecules.
- Washing: Wash the membrane to remove unbound probes, ensuring specificity in detection.
- Signal Detection: Detect the presence of bound probes through techniques like autoradiography, chemiluminescence, or fluorescence.
- Analysis: Analyze the results to determine the presence, quantity, or characteristics of the target biomolecules.
- Documentation: Document the results, often by capturing images of the membrane to record the data.
- Interpretation: Interpret the data obtained from the reverse blotting analysis, drawing conclusions based on the detected signals.
- Applications: Utilize the results for various applications, such as gene expression analysis, protein detection, or clinical diagnostics.
Materials and Reagents:
- Agarose or Polyacrylamide Gel: To separate biomolecules.
- Solid Membrane (e.g., Nitrocellulose or PVDF): For biomolecule transfer and immobilization.
- Transfer Apparatus: Such as a semi-dry or wet transfer system.
- Transfer Buffer: To maintain appropriate pH and ionic strength during transfer.
- Staining Dye or Label: For gel visualization.
- Fixation Solution: To immobilize biomolecules on the membrane.
- Probe: Labeled DNA, RNA, or antibodies specific to target biomolecules.
- Washing Buffers: To remove unbound probes.
- Detection Substrates: Such as chemiluminescent or fluorescent substrates.
- Autoradiography Film: For signal visualization in radioactive probing.
- Chemiluminescence Detection System: If using chemiluminescent probes.
- Fluorescence Imaging System: If using fluorescent probes.
- UV Cross-Linker: For fixation in UV cross-linking.
- Laboratory Consumables: Such as pipettes, tubes, and gel cassettes.
- Safety Equipment: Including gloves, lab coats, and protective eyewear.
Step-by-Step Protocol:
- Prepare Gel: Perform gel electrophoresis to separate biomolecules based on size or charge.
- Stain Gel: Stain the gel to visualize biomolecules and wash off excess stain.
- Set Up Transfer Apparatus: Assemble the reverse blotting apparatus with a transfer buffer tank and a solid membrane.
- Gel-Membrane Sandwich: Place the gel onto the membrane, ensuring no air bubbles.
- Apply Electric Field: Connect the gel and membrane to the transfer apparatus to facilitate biomolecule transfer.
- Transfer Buffer: Circulate transfer buffer between the gel and membrane during transfer.
- Fixation: After transfer, fix biomolecules on the membrane (UV cross-link or heat).
- Probe Incubation: Incubate membrane with labeled DNA, RNA, or antibodies for target detection.
- Wash Membrane: Remove unbound probes with washing steps.
- Signal Detection: Detect bound probes using appropriate detection methods.
- Analysis: Analyze results to determine target presence, quantity, or characteristics.
- Documentation: Capture images or record data for documentation.
- Interpretation: Interpret results to draw conclusions based on detected signals.
- Applications: Utilize results for gene expression analysis, protein detection, or diagnostics.
Result Interpretation:
Result interpretation in reverse blotting involves analyzing the data obtained from the blotting procedure to draw meaningful conclusions about the presence, quantity, or characteristics of the target biomolecules. Here are some key points for result interpretation in reverse blotting:
- Signal Detection: Begin by examining the signals detected on the membrane. These signals indicate the presence of the target biomolecules.
- Signal Intensity: Assess the intensity of the signals. Stronger signals typically indicate a higher quantity of the target molecules.
- Position of Bands: Determine the position of the signal bands on the membrane. This can provide information about the size or molecular weight of the biomolecules.
- Control Samples: Compare the signals in the experimental samples with those in control samples. Control samples can include negative controls (no target) and positive controls (known targets).
- Quantification: If applicable, use quantitative data obtained from the blot, such as signal intensity or band density, to quantify the amount of the target molecules present.
- Normalization: Normalize the data if necessary. This involves comparing the signal of the target molecule to a reference signal, often from a loading control or internal standard.
- Comparison: Compare the results of different samples or experimental conditions. Look for differences or similarities in the presence or quantity of target molecules.
- Specificity: Assess the specificity of the signals. Ensure that the signals are specific to the target molecules and not due to non-specific binding.
- Replicates: If you have performed multiple replicates of the experiment, assess the consistency of results across replicates.
- Background Noise: Be aware of any background noise or non-specific signals that may interfere with result interpretation. Proper controls and washing steps can help reduce background noise.
- Pattern Recognition: In some cases, patterns of signals (e.g., multiple bands or shifts) can provide insights into post-translational modifications or alternative splicing events.
- Correlation with Hypotheses: Interpret the results in the context of your research hypotheses or objectives. Do the results support or contradict your initial hypotheses?
- Biological Relevance: Consider the biological significance of the findings. How do the results relate to the broader biological or clinical questions you are investigating?
- Data Reporting: Accurately report and document the results, including any statistical analyses performed, in a clear and organized manner.
- Conclusions: Based on your interpretation, draw conclusions about the presence, quantity, or characteristics of the target biomolecules. Discuss the implications of your findings and their relevance to the research objectives.
- Further Experiments: Depending on the results, consider whether further experiments or follow-up studies are needed to validate or expand upon your findings.
Result interpretation in reverse blotting is a critical step in the research process, as it allows you to derive meaningful insights and advance your understanding of the biological or biochemical systems you are studying.
Troubleshooting and Tips:
1. Inefficient Transfer:
- Issue: Weak or no signal on the membrane.
- Troubleshooting:
- Check for proper contact between the gel and the membrane.
- Verify that the transfer apparatus is functioning correctly.
- Ensure the transfer buffer has the correct pH and ionic strength.
- Extend the transfer time or increase the voltage if necessary.
2. Incomplete Transfer:
- Issue: Bands are visible on the gel but not on the membrane.
- Troubleshooting:
- Confirm that the gel is well-saturated with transfer buffer.
- Ensure there are no air bubbles trapped between the gel and the membrane.
- Optimize the transfer conditions (buffer composition, time, voltage).
3. High Background Noise:
- Issue: Excessive non-specific signals on the membrane.
- Troubleshooting:
- Improve blocking with solutions like BSA or milk.
- Ensure thorough washing to remove unbound probes.
- Reduce probe concentration if necessary.
- Opt for more specific primary and secondary antibodies or probes.
4. Faint or Weak Signals:
- Issue: Signals are present but appear faint.
- Troubleshooting:
- Increase probe concentration or optimize labeling efficiency.
- Check the quality and freshness of detection reagents.
- Extend exposure time during signal detection.
5. Irregular Band Patterns:
- Issue: Bands exhibit unusual shapes or patterns.
- Troubleshooting:
- Verify that the gel and membrane are correctly aligned.
- Ensure even pressure during the transfer process.
- Check for irregularities in the gel or membrane.
6. Membrane Damage:
- Issue: Membrane tearing or damage during transfer.
- Troubleshooting:
- Handle the membrane carefully to prevent physical damage.
- Ensure that the gel is evenly placed on the membrane to avoid tears.
7. Poor Probe Binding:
- Issue: Low or no probe binding to target biomolecules.
- Troubleshooting:
- Confirm that probes are specific to the target sequences.
- Optimize hybridization conditions (temperature, time, buffer).
- Consider pre-blocking the membrane with blocking agents.
8. Reproducibility Issues:
- Issue: Inconsistent results across replicates.
- Troubleshooting:
- Standardize and document all steps of the protocol.
- Pay attention to consistent buffer preparation and handling.
- Ensure proper control samples are included.
9. Insufficient Documentation:
- Issue: Poor record-keeping leading to difficulties in result interpretation.
- Troubleshooting:
- Maintain a detailed lab notebook with all experimental details.
- Label gels, membranes, and samples clearly.
- Document instrument settings, probe concentrations, and exposure times.
10. Quality Control:
- Tip: Regularly perform quality control checks, including positive and negative controls, to ensure the reliability of your reverse blotting experiments.
Advantages and Disadvantages of Reverse Blotting:
Advantages:
- Preservation of Sample Integrity: Reverse blotting allows the preservation of biomolecules separated in the gel, enabling multiple analyses from a single gel.
- Versatility: It can be used for DNA (Southern blotting), RNA (Northern blotting), and protein (Western blotting) analyses, making it suitable for various biomolecules.
- Quantification: It permits quantitative analysis of target molecules by comparing signal intensities to standards.
- High Sensitivity: It is capable of detecting even low-abundance biomolecules.
- Specificity: Specific probes or antibodies can be used for target molecule detection, enhancing specificity.
- Multiplexing: Multiple target molecules can be detected on a single membrane by using different probes or antibodies.
- Long-Term Storage: Blotted membranes can be stored for an extended period, allowing future reanalysis.
- Clinical and Diagnostic Applications: Reverse blotting is widely used in clinical laboratories for disease diagnosis and monitoring.
Disadvantages:
- Time-Consuming: The technique involves multiple steps, including gel electrophoresis, transfer, probing, and detection, which can be time-consuming.
- Complexity: The method requires expertise and precision to avoid issues like incomplete transfer or high background noise.
- Protein Denaturation: In Western blotting, proteins may become denatured during the transfer process, affecting the detection of native protein structures.
- Expense: The reagents and materials for reverse blotting, including membranes and labeled probes, can be costly.
- Limited Sample Throughput: Reverse blotting is typically performed on a small number of samples at a time, making it less suitable for high-throughput applications.
- Variability: Results can vary between experiments due to factors like gel quality, transfer efficiency, or probe performance.
- Labor-Intensive: The technique requires careful handling of gels and membranes, increasing the potential for human error.
- Detection Limitations: Sensitivity can be limited in cases of extremely low-abundance target molecules.
Limitations of Reverse Blotting:
- Time-Consuming: The technique involves multiple steps, making it time-consuming.
- Complexity: Requires expertise and precision to avoid issues like incomplete transfer or high background noise.
- Protein Denaturation: In Western blotting, proteins may become denatured during transfer, affecting detection of native protein structures.
- Expense: Reagents and materials can be costly, especially for multiple samples.
- Limited Sample Throughput: Typically suited for a small number of samples, not ideal for high-throughput applications.
- Variability: Results may vary between experiments due to factors like gel quality or probe performance.
- Sensitivity Limitation: Limited sensitivity for extremely low-abundance target molecules.
- Labor-Intensive: Requires careful handling, increasing the potential for human error.
Variations and Modern Alternatives:
Variations:
- Dot Blotting: Similar to reverse blotting, but samples are applied directly to a membrane in a spotted format, making it suitable for rapid screening.
- Slot Blotting: A modification of dot blotting, where samples are aspirated through slots onto the membrane, providing semi-quantitative results.
- RNA Blotting (Northern Blotting): Specifically designed for RNA analysis, it involves the transfer and detection of RNA molecules from a gel to a membrane.
- DNA Blotting (Southern Blotting): Used for DNA analysis, particularly in the detection of specific DNA sequences or genetic mutations.
- Western Blotting: Focuses on the transfer and detection of proteins from a gel to a membrane, allowing analysis of protein size, abundance, and post-translational modifications.
Modern Alternatives:
- PCR-Based Techniques: Polymerase chain reaction (PCR) and its variants like qPCR and RT-PCR enable the amplification and detection of DNA and RNA sequences without the need for blotting.
- Next-Generation Sequencing (NGS): NGS techniques provide high-throughput analysis of DNA and RNA, allowing comprehensive genomics and transcriptomics studies.
- Mass Spectrometry: MS-based proteomics techniques offer highly sensitive and quantitative analysis of proteins, providing insights into protein identification and quantification.
- Fluorescence-Based Assays: Fluorescent probes and dyes are used for direct detection of specific biomolecules, offering rapid and sensitive alternatives to blotting.
- Microarrays: Microarray technology allows simultaneous analysis of thousands of DNA, RNA, or protein targets, making it suitable for high-throughput studies.
- ELISA (Enzyme-Linked Immunosorbent Assay): ELISA is used for protein detection and quantification in a microplate format, offering high sensitivity and specificity.
- Immunohistochemistry (IHC): Used for protein detection in tissue sections, IHC utilizes antibodies and chromogenic or fluorescent detection methods.
- RNA Sequencing (RNA-Seq): Provides comprehensive transcriptome analysis, including differential gene expression and alternative splicing, without the need for Northern blotting.
- Digital PCR (dPCR): Offers absolute quantification of DNA or RNA targets, reducing the need for gel electrophoresis and Southern or Northern blotting.
- Western Blot Alternatives: Various alternatives, including immunoblotting, proximity ligation assays, and antibody arrays, provide more streamlined and specific protein analysis methods.
Comparison of Reverse Blotting with Modern Techniques:
| Aspect | Reverse Blotting | PCR-Based Techniques | Next-Generation Sequencing (NGS) | Mass Spectrometry |
|---|---|---|---|---|
| Principle | Transfer and detection of biomolecules from a gel to a membrane for analysis. | Amplification and detection of specific DNA or RNA sequences. | High-throughput sequencing of DNA or RNA for genomics or transcriptomics. | Identification and quantification of proteins or small molecules based on mass-to-charge ratios. |
| Sample Type | DNA, RNA, or proteins separated in a gel. | DNA, RNA, or specific genes of interest. | Genomic DNA, cDNA, or RNA from various sources. | Proteins, peptides, or metabolites in complex mixtures. |
| Throughput | Low to moderate throughput, typically analyzing a small number of samples at once. | Moderate to high throughput, capable of analyzing many samples simultaneously. | High throughput, capable of analyzing thousands to millions of sequences in parallel. | Low to moderate throughput, depending on the instrument. |
| Sensitivity | High sensitivity for target molecules. | High sensitivity for DNA or RNA targets, especially qPCR. | High sensitivity for detecting rare mutations or low-abundance sequences. | High sensitivity for detecting low-abundance proteins or metabolites. |
| Specificity | Highly specific when using specific probes or antibodies. | Highly specific with the use of primer design and specificity controls. | High specificity due to sequencing and bioinformatic analysis. | High specificity through mass spectrometry-based identification. |
| Quantification | Quantitative with proper calibration and standardization. | Quantitative, often with absolute quantification capabilities. | Quantitative, enabling gene expression analysis and more. | Quantitative, providing abundance data for identified molecules. |
| Application Range | Suitable for DNA, RNA, and protein analysis in a laboratory setting. | Suitable for various DNA or RNA assays, including diagnostics. | Widely used in genomics, transcriptomics, and epigenomics research. | Commonly used in proteomics, metabolomics, and drug discovery. |
| Time and Cost | Time-consuming, moderate cost for reagents and materials. | Time-efficient, moderate to high cost depending on assays and equipment. | Time-efficient for high throughput, cost varies with sequencing depth. | Time-consuming, cost varies with instrument and analysis complexity. |
| Ease of Use | Requires expertise, may involve multiple steps. | User-friendly, accessible to non-specialists. | Requires bioinformatics expertise for data analysis. | Requires expertise in mass spectrometry and data analysis. |
| Limitations | Limited throughput, longer processing time, potential for high background. | PCR bias, limited to known sequences, may require prior knowledge. | Limited to detecting sequences present in the sample, potential for bias. | Limited ability to identify unknown molecules, may require complex sample preparation. |
FAQs:
1. What is reverse blotting?
- Reverse blotting is a laboratory technique used in molecular biology to transfer biomolecules (DNA, RNA, or proteins) from a gel onto a solid membrane for subsequent analysis and detection.
2. What are the key applications of reverse blotting?
- Reverse blotting is used in applications like Southern blotting (for DNA), Northern blotting (for RNA), Western blotting (for proteins), and other molecular biology assays. It is employed in gene expression analysis, protein detection, clinical diagnostics, and more.
3. How does reverse blotting differ from traditional blotting?
- In reverse blotting, biomolecules are transferred from a gel to a membrane (opposite direction) for analysis, whereas traditional blotting transfers molecules from a membrane to a gel.
4. What types of membranes are commonly used in reverse blotting?
- Common membrane types include nitrocellulose and PVDF (polyvinylidene difluoride), both of which have high binding capacity for biomolecules.
5. What are the advantages of using reverse blotting?
- Advantages include the preservation of separated biomolecules, versatility for DNA, RNA, and protein analysis, quantification capabilities, and high sensitivity for target detection.
6. What are the limitations of reverse blotting?
- Limitations include its time-consuming nature, complexity, potential for protein denaturation in Western blotting, cost of reagents and materials, and limited throughput for a small number of samples.
7. How can I troubleshoot common issues in reverse blotting?
- Common issues include inefficient transfer, incomplete transfer, high background noise, and more. Troubleshooting steps include checking for proper contact, buffer conditions, probe quality, and precise handling.
8. Are there modern alternatives to reverse blotting?
- Yes, modern alternatives include PCR-based techniques (PCR, qPCR, RT-PCR), next-generation sequencing (NGS), mass spectrometry, microarrays, and fluorescence-based assays. These offer different advantages and throughput levels for biomolecule analysis.
9. How do I choose between reverse blotting and alternative techniques?
- The choice depends on your research goals, the type of biomolecules you’re analyzing, throughput requirements, sensitivity needs, and available resources. Each technique has its strengths and limitations.
10. Is reverse blotting still commonly used in research today?
- Yes, reverse blotting remains a valuable and widely used technique in molecular biology and biochemistry, especially in applications where preserving and analyzing separated biomolecules are critical.
Conclusion:
In conclusion, reverse blotting is a versatile and valuable laboratory technique in molecular biology and biochemistry. It allows for the transfer of biomolecules (DNA, RNA, or proteins) from a gel to a solid membrane, facilitating their analysis and detection. Reverse blotting finds applications in various research areas, including gene expression analysis, protein detection, clinical diagnostics, and environmental monitoring.
While reverse blotting offers advantages such as the preservation of sample integrity, specificity, and quantification capabilities, it also has limitations, including its time-consuming nature and limited throughput for a small number of samples.
Researchers have the option to choose from modern alternatives, such as PCR-based techniques, next-generation sequencing, and mass spectrometry, depending on their specific research goals, sample types, and resources.
Possible References Used