Why Are Regulatory Affairs Essential in Clinical Trials?
Regulatory affairs play a crucial role in the success of clinical trials by ensuring that research complies with legal and ethical standards. Every clinical trial must adhere to guidelines set by regulatory bodies such as the FDA, EMA, and national health agencies. These regulations are designed to protect patient safety, maintain data integrity, and ensure that investigational products undergo rigorous evaluation before entering the market.
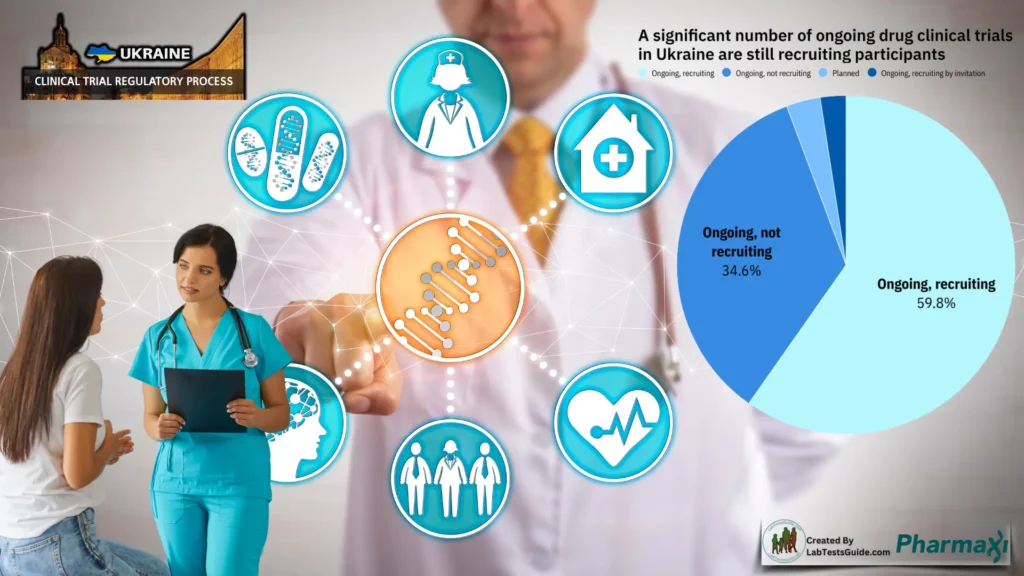
Without proper regulatory oversight, clinical trials in Ukraine risk delays, rejection of results, or even legal consequences. The complexity of compliance requirements makes regulatory affairs professionals essential in guiding sponsors through the approval process and helping them meet global regulatory expectations.
What Are the Key Regulatory Challenges in Conducting Clinical Trials in Ukraine?
Ukraine has emerged as an attractive location for clinical research due to its skilled workforce, cost-effective operations, and increasing alignment with international regulatory standards. However, sponsors and Contract Research Organizations (CROs) must navigate various regulatory challenges, such as:
- Harmonization with International Guidelines: While Ukraine follows Good Clinical Practice (GCP) guidelines, ongoing regulatory updates are required to align with evolving EU and FDA standards.
- Ethics Committee Approvals: Clinical trials in Ukraine must receive approval from ethics committees, which can sometimes result in delays due to procedural requirements.
- Import and Export Regulations: Strict policies regarding the import of investigational drugs and export of biological samples require careful compliance to avoid disruptions.
- Data Protection Laws: As Ukraine strengthens its data privacy regulations, clinical trials must adhere to updated patient data protection requirements to avoid compliance risks.
The Role of Regulatory Affairs in Clinical Trials
Effective regulatory affairs strategies involve multiple responsibilities, including:
- Regulatory Submissions: Preparing and submitting clinical trial applications to health authorities for approval.
- Compliance Monitoring: Ensuring trials follow Good Clinical Practice (GCP) and International Council for Harmonisation (ICH) guidelines.
- Risk Management: Identifying potential regulatory risks and implementing measures to mitigate them.
- Communication with Authorities: Maintaining clear and transparent interactions with regulatory agencies to facilitate approvals.
Benefits of Strong Regulatory Compliance
A well-managed regulatory affairs process provides several advantages:
- Faster approval timelines for clinical trials.
- Reduced risk of protocol violations and study termination.
- Improved trust and collaboration with regulatory authorities.
- Higher acceptance of trial data by global regulatory agencies.
Clinical Trials in Ukraine: Regulatory Advantages
Despite regulatory challenges, Ukraine offers several benefits for conducting clinical trials, including:
- Expedited Approval Processes: Recent reforms in Ukraine’s regulatory framework have streamlined approval timelines for clinical trial applications.
- Experienced Investigators and Research Sites: Ukraine has a well-trained medical research community, ensuring high-quality trial execution.
- Cost-Effective Trial Operations: Lower costs for patient recruitment, site management, and data processing make Ukraine a competitive option for sponsors.
- Patient Availability: A large population with diverse medical conditions enables faster recruitment of trial participants.
Best Practices for Ensuring Regulatory Compliance in Clinical Trials
To successfully navigate regulatory challenges, sponsors and CROs should implement the following best practices:
- Engage Regulatory Experts Early: Involving regulatory affairs professionals at the planning stage helps identify potential hurdles and ensures smoother approval processes.
- Develop Comprehensive Regulatory Strategies: A well-defined strategy should address submission timelines, approval requirements, and post-approval monitoring.
- Maintain Thorough Documentation: Keeping accurate records of regulatory submissions, ethics approvals, and trial protocols is essential for compliance audits.
- Stay Updated on Regulatory Changes: Continuous monitoring of evolving regulations ensures that clinical trials in Ukraine remain compliant with new requirements.
The Future of Regulatory Affairs in Clinical Trials
As regulatory landscapes evolve, several emerging trends are shaping the future of regulatory affairs in clinical research:
- Digitalization of Regulatory Processes: Electronic submissions and remote audits are streamlining compliance workflows.
- Harmonization of Global Regulations: Efforts to standardize regulatory requirements across regions will facilitate international trial approvals.
- AI-Driven Regulatory Analytics: Artificial intelligence is enhancing regulatory decision-making by identifying compliance risks and optimizing submission strategies.
- Increased Focus on Patient-Centric Regulations: Regulatory agencies are prioritizing patient engagement and transparency in clinical trial processes.
Effective regulatory affairs management is critical for the success of clinical trials in Ukraine and globally. By implementing best practices and staying informed about regulatory developments, sponsors and CROs can navigate compliance challenges while ensuring the ethical and efficient conduct of clinical research.
Possible References Used