Northern blotting, also known as RNA blotting, is a laboratory technique used in molecular biology to study gene expression by detecting and analyzing RNA molecules in a sample. It is named after its counterpart, Southern blotting, which is used to study DNA molecules. Northern blotting allows researchers to determine the size and abundance of specific RNA molecules, as well as to assess their expression patterns under different conditions.
Northern blotting, also known as RNA blotting, is a molecular biology technique used to study gene expression by detecting RNA in a sample. It is named after Edwin Southern, who developed a similar technique for detecting DNA.
Key points of Northern Blotting:
- RNA Analysis: Northern blotting is a technique for analyzing RNA molecules.
- Gene Expression: It helps researchers study gene expression by detecting specific RNAs.
- DNA Counterpart: Named after Southern blotting, which is used for DNA analysis.
- RNA Size and Abundance: Northern blotting reveals the size and abundance of RNA.
- RNA Extraction: It starts with extracting RNA from a sample.
- Denaturation: RNA is heated to make it single-stranded.
- Gel Electrophoresis: RNA is separated by size using a gel.
- Gel Types: Can use agarose or polyacrylamide gels.
- Migration: Smaller RNA moves faster through the gel.
- Membrane Transfer: RNA is transferred to a solid membrane (nitrocellulose or nylon).
- Immobilization: RNA is fixed or immobilized on the membrane.
- Hybridization: A complementary labeled probe is used to detect target RNA.
- Probe Labels: Probes can be labeled with radioisotopes, dyes, or enzymes.
- Wash Step: Unbound probe is washed away after hybridization.
- Detection Methods: Bound probe is detected using various methods (autoradiography, chemiluminescence, or fluorescence).
- Information Gained: Northern blotting provides data on RNA size and quantity.
- Sensitivity: It’s less sensitive than newer RNA analysis methods like qRT-PCR and RNA-Seq.
- Continued Use: Northern blotting is still used for precise RNA quantification and size determination.
Defination of Northern Blotting:
Northern blotting is a molecular biology technique used to analyze and detect specific RNA molecules in a sample, providing information about their size and abundance.
Background and Significance:
- RNA Analysis: Northern blotting is a classic method developed in the 1970s to study RNA molecules, allowing researchers to gain insights into gene expression.
- Gene Expression: It is a crucial tool for investigating gene expression patterns under various conditions, providing valuable information about which genes are active and to what extent.
- Predecessor to Modern Techniques: Northern blotting paved the way for more advanced RNA analysis techniques like RT-PCR, qRT-PCR, and RNA-Seq, which are now widely used but built upon the principles of Northern blotting.
- Size Determination: Northern blotting provides precise information about the size of RNA molecules, aiding in the identification of specific RNA species.
- Abundance Measurement: Researchers can estimate the abundance of RNA molecules, allowing for comparisons of gene expression levels between different samples.
- Biomedical Research: In biomedical research, Northern blotting has been instrumental in understanding the role of specific genes in diseases and conditions.
- Validation Tool: It is used as a validation tool for confirming the results obtained from newer, high-throughput RNA analysis methods.
- Historical Significance: Northern blotting, along with Southern blotting (for DNA analysis), revolutionized molecular biology and genetic research.
- Diagnostic Applications: In clinical settings, Northern blotting has been used for diagnosing certain genetic disorders by detecting abnormal RNA patterns.
- Teaching and Education: Northern blotting is often taught in molecular biology and genetics courses as it provides fundamental insights into RNA analysis techniques.
Purpose of Northern Blotting:
- RNA Detection: To detect and confirm the presence of specific RNA molecules in a given sample.
- Gene Expression Analysis: To study and quantify gene expression levels under different experimental conditions.
- Size Determination: To determine the size of RNA molecules and verify their identity.
- Abundance Measurement: To estimate the relative abundance of specific RNA transcripts.
- Comparative Studies: To compare RNA expression patterns between different tissues, developmental stages, or experimental treatments.
- RNA Characterization: To characterize RNA species, such as mRNA, rRNA, tRNA, and small regulatory RNAs.
- Validation: To validate results obtained from other RNA quantification methods like qRT-PCR or RNA-Seq.
- Research Tool: As a fundamental tool in molecular biology research for understanding gene regulation and RNA biology.
- Clinical Applications: In diagnosing certain genetic disorders where abnormal RNA patterns are indicative of the condition.
- Teaching and Education: As a teaching tool to illustrate principles of RNA analysis and gene expression to students in molecular biology and genetics courses.
Applications of Northern Blotting:
- Gene Expression Analysis: Northern blotting is used to assess the expression levels of specific genes under different experimental conditions. Researchers can determine which genes are active and to what extent.
- mRNA Detection: It is commonly employed to detect and quantify messenger RNA (mRNA) molecules, providing insights into the transcriptional activity of genes.
- Alternative Splicing: Researchers use Northern blotting to investigate alternative splicing patterns of mRNA, which can result in different protein isoforms.
- RNA Size Determination: It allows for precise determination of the sizes of RNA molecules, aiding in the identification of specific transcripts.
- Studying Non-coding RNAs: Northern blotting can be used to study non-coding RNAs such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs).
- Comparative Analysis: Researchers can compare RNA expression patterns between different tissues, developmental stages, or experimental conditions to understand gene regulation.
- Quality Control: It serves as a quality control method to confirm the integrity of RNA samples before proceeding with downstream applications.
- Validation of RNA-seq Data: Northern blotting can validate results obtained from high-throughput techniques like RNA sequencing (RNA-seq).
- Diagnostic Tool: In clinical settings, Northern blotting has been used to diagnose certain genetic disorders by detecting abnormal RNA patterns associated with these conditions.
- Pharmaceutical Research: It plays a role in pharmaceutical research, particularly in assessing the effects of drugs or treatments on gene expression.
- Teaching and Education: Northern blotting is used as a teaching tool to demonstrate the principles of RNA analysis and gene expression regulation in educational settings.
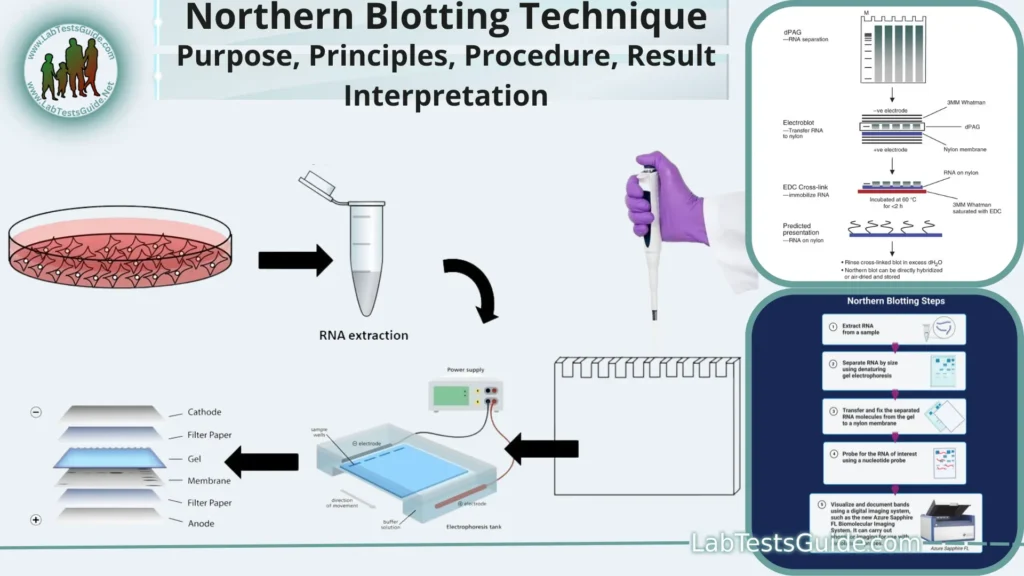
Principles of Northern Blotting:
- RNA Separation by Size: Northern blotting begins with the separation of RNA molecules based on their size. RNA samples are typically run on an agarose or polyacrylamide gel, where smaller RNA molecules migrate more quickly through the gel matrix than larger ones. This separation is achieved through electrophoresis.
- RNA Transfer to a Membrane: After electrophoresis, the separated RNA molecules are transferred from the gel onto a solid support membrane, such as nitrocellulose or nylon. This transfer can be done via capillary action or electroblotting, effectively immobilizing the RNA on the membrane.
- RNA Fixation: The transferred RNA is then fixed or immobilized onto the membrane using methods such as UV cross-linking, heat treatment, or chemical cross-linking. This step ensures that the RNA remains in place for subsequent analysis.
- Hybridization: The immobilized RNA on the membrane is exposed to a specific nucleic acid probe that is complementary to the RNA of interest. This probe is typically labeled with a detectable marker, such as a radioactive isotope (e.g., 32P), a fluorescent dye, or an enzyme (e.g., alkaline phosphatase or horseradish peroxidase). The probe hybridizes or binds to the complementary RNA sequence on the membrane.
- Washing: After hybridization, the membrane is washed to remove any unbound probe molecules. This step helps reduce background noise and increases the specificity of detection.
- Probe Detection: The bound probe is then detected using a method appropriate for the probe label. For radioactive probes, autoradiography is used to visualize the position and intensity of the signals. Enzyme-labeled probes can be detected using substrates that produce a visible signal, while fluorescently labeled probes are visualized using a fluorescent imaging system.
- Signal Analysis: The resulting image or signal on the membrane is analyzed to determine the presence and abundance of the specific RNA molecules. This analysis provides information about the size and quantity of the RNA of interest.
- Controls: To ensure the accuracy and reliability of results, controls are typically included, such as positive controls (known RNA samples) and negative controls (samples without the RNA of interest).
Procedure for Northern Blotting:
Performing a Northern blotting experiment involves several steps. Here’s a general procedure for Northern blotting:
Materials and Equipment:
- RNA samples of interest
- Agarose or polyacrylamide gel
- Nitrocellulose or nylon membrane
- Buffer solutions (e.g., denaturation buffer, electrophoresis buffer)
- Ethidium bromide or other gel-staining dye
- RNA size markers
- Radiolabeled or labeled DNA or RNA probe
- Hybridization buffer
- Wash solutions
- Autoradiography film (if using radioactive probes)
- X-ray film developer (if using radioactive probes)
- Darkroom or chemiluminescence imaging system (if using enzyme-labeled probes)
- Exposure box (if using fluorescent probes)
- Appropriate safety precautions for handling radioactive materials (if applicable)
Procedure:
- RNA Extraction and Denaturation:
- Extract RNA from your biological sample using a suitable RNA extraction method.
- Treat the extracted RNA with denaturation buffer (usually containing formaldehyde) to denature the RNA molecules and break secondary structures. Heat the mixture at 65-70°C for 10-15 minutes.
- Gel Electrophoresis:
- Prepare an agarose or polyacrylamide gel, depending on the size range of your RNA molecules.
- Mix the denatured RNA samples with loading buffer and load them onto the gel.
- Run the gel under an electric field, with smaller RNA migrating faster through the gel.
- Stain the gel with ethidium bromide and visualize the RNA bands under UV light to confirm RNA integrity and size separation.
- Transfer to Membrane:
- Cut a piece of nitrocellulose or nylon membrane to match the size of your gel.
- Soak the membrane in transfer buffer.
- Assemble a gel transfer sandwich: gel, membrane, filter paper, and sponge. Apply gentle pressure or use a transfer apparatus to transfer the RNA from the gel to the membrane (usually via capillary or electroblotting). The RNA should bind to the membrane.
- Membrane Fixation:
- Fix the RNA to the membrane, usually by UV cross-linking or baking at 80-120°C for a short time.
- Probe Preparation:
- Prepare a labeled DNA or RNA probe specific to your RNA of interest. The probe can be labeled with radioisotopes, fluorescent tags, or enzymes (e.g., alkaline phosphatase or horseradish peroxidase).
- Hybridization:
- Prehybridize the membrane in hybridization buffer to reduce nonspecific binding.
- Incubate the membrane with your labeled probe in hybridization buffer under appropriate conditions (e.g., temperature and time).
- The probe will hybridize to complementary RNA sequences on the membrane.
- Washing:
- Wash the membrane with stringency buffers to remove unbound probe, which reduces background noise and increases specificity.
- Probe Detection:
- Detect the probe on the membrane using the appropriate method for your labeling system.
- Autoradiography for radioactive probes, chemiluminescence or colorimetry for enzyme-labeled probes, or fluorescence for fluorescently labeled probes.
- Analysis:
- Analyze the Northern blot results, typically by quantifying signal intensity to determine the abundance of specific RNA molecules.
- Controls and Documentation:
- Include positive and negative controls to validate your results.
- Document the results by taking photographs or using imaging equipment.
- Data Interpretation:
- Interpret the data to draw conclusions about the expression levels and sizes of RNA molecules in your samples.
Materials and Reagents:
Materials:
- RNA Samples: Your RNA samples of interest, extracted from cells, tissues, or other biological sources.
- Agarose or Polyacrylamide Gel: To separate RNA molecules based on size.
- Nitrocellulose or Nylon Membrane: For transferring RNA from the gel and immobilizing it for subsequent analysis.
- Buffer Solutions: Various buffer solutions are required, including:
- Denaturation buffer: To denature RNA samples.
- Electrophoresis buffer: To run the gel.
- Transfer buffer: For transferring RNA to the membrane.
- Hybridization buffer: To facilitate probe binding.
- Wash solutions: For removing unbound probe.
- Ethidium Bromide or Gel Staining Dye: For visualizing RNA bands on the gel.
- RNA Size Markers: Commercially available RNA markers with known sizes to estimate RNA molecule sizes.
- Radiolabeled or Labeled Probes: DNA or RNA probes specific to your target RNA, labeled with radioactive isotopes (e.g., 32P), fluorescent tags, or enzymes for detection.
- Autoradiography Film: If using radioactive probes, this film captures the signals.
- X-ray Film Developer: Required to develop autoradiography film.
- Darkroom or Chemiluminescence Imaging System: For visualizing signals if using enzyme-labeled probes.
- Exposure Box: If using fluorescently labeled probes, this is used to expose the membrane to a fluorescence imaging system.
Reagents:
- RNA Extraction Reagents: Reagents and kits for RNA extraction, such as TRIzol, phenol-chloroform, or commercial RNA isolation kits.
- Formaldehyde: Used in denaturation buffer to denature RNA.
- Loading Buffer: To prepare RNA samples for gel electrophoresis.
- Transfer Buffer: For transferring RNA from the gel to the membrane.
- Hybridization Buffer: Contains components for optimal probe binding during hybridization.
- Blocking Agent: Such as bovine serum albumin (BSA) or non-fat dry milk, used to reduce nonspecific binding of the probe.
- Salts and Detergents: Included in various buffers and solutions to maintain proper conditions for hybridization and washing steps.
- Stripping Buffer: If you plan to reuse the membrane, stripping buffer is used to remove the bound probe.
- RNAse Inhibitor: To prevent RNA degradation during various steps of the procedure.
- Sodium Dodecyl Sulfate (SDS): Included in some buffers to denature RNA and proteins.
- Tris and Acetic Acid (TAE) Buffer: Often used in electrophoresis and gel preparation.
- Molecular Biology-Grade Water: For preparing solutions and dilutions.
- Disposable Gloves and Laboratory Supplies: Pipettes, tubes, filter papers, and other basic lab supplies for handling samples and reagents.
Step-by-Step Protocol:
Materials and Reagents:
- RNA samples
- Agarose or polyacrylamide gel
- Nitrocellulose or nylon membrane
- Denaturation buffer
- Electrophoresis buffer
- Transfer buffer
- Ethidium bromide or gel staining dye
- RNA size markers
- Radiolabeled DNA or RNA probe
- Autoradiography film
- X-ray film developer
- Darkroom
- Exposure box
- RNA extraction reagents
- Formaldehyde
- Loading buffer
- Hybridization buffer
- Blocking agent (e.g., non-fat dry milk)
- Salts and detergents (e.g., SDS)
- Stripping buffer
- RNAse inhibitor
- Sodium dodecyl sulfate (SDS)
- Tris and Acetic Acid (TAE) buffer
- Molecular biology-grade water
- Disposable gloves and laboratory supplies
Procedure:
- RNA Extraction and Denaturation:
- Extract RNA from your biological sample using a suitable RNA extraction method.
- Treat the extracted RNA with denaturation buffer (containing formaldehyde) to denature the RNA molecules. Heat the mixture at 65-70°C for 10-15 minutes.
- Gel Preparation:
- Prepare an agarose or polyacrylamide gel depending on the size range of your RNA molecules.
- Mix denatured RNA samples with loading buffer and load them onto the gel.
- Run the gel under an electric field, with smaller RNA migrating faster through the gel.
- Stain the gel with ethidium bromide or a gel staining dye and visualize the RNA bands under UV light to confirm RNA integrity and size separation.
- Transfer to Membrane:
- Cut a piece of nitrocellulose or nylon membrane to match the size of your gel.
- Soak the membrane in transfer buffer.
- Assemble a gel transfer sandwich: gel, membrane, filter paper, and sponge. Apply gentle pressure or use a transfer apparatus to transfer the RNA from the gel to the membrane (usually via capillary or electroblotting). The RNA should bind to the membrane.
- Membrane Fixation:
- Fix the RNA to the membrane, usually by UV cross-linking or baking at 80-120°C for a short time.
- Probe Preparation:
- Prepare a radiolabeled DNA or RNA probe specific to your target RNA.
- Hybridization:
- Prehybridize the membrane in hybridization buffer to reduce nonspecific binding.
- Incubate the membrane with your radiolabeled probe in hybridization buffer under appropriate conditions (e.g., temperature and time). The probe will hybridize to complementary RNA sequences on the membrane.
- Washing:
- Wash the membrane with stringency buffers to remove unbound probe, reducing background noise and increasing specificity.
- Autoradiography:
- Expose the membrane to an autoradiography film in a darkroom with an exposure box.
- Develop the film using an X-ray film developer.
- Analysis:
- Analyze the autoradiography film to determine the presence and abundance of specific RNA molecules.
- Controls and Documentation:
- Include positive and negative controls to validate your results.
- Document the results by taking photographs or using imaging equipment.
- Data Interpretation:
- Interpret the data to draw conclusions about the expression levels and sizes of RNA molecules in your samples.
Result Interpretation:
- Visual Inspection:
- Examine the autoradiograph (or other detection method) for the presence of bands. Each band represents a specific RNA molecule.
- Note the position of each band on the membrane.
- Size Determination:
- Use the RNA size markers loaded alongside your samples on the gel to estimate the size of each RNA band. Compare the migration distance of the bands to the known sizes of the markers.
- You can create a standard curve using the size markers to estimate the sizes of unknown RNA bands accurately.
- Abundance Assessment:
- Assess the intensity or darkness of the bands on the autoradiograph. A darker band indicates a higher abundance of the corresponding RNA molecule.
- Quantify the signal intensity using appropriate software or tools, if necessary, to obtain semi-quantitative or quantitative data on RNA abundance.
- Sample Comparison:
- Compare the RNA bands between different samples or conditions. Differences in band intensity or presence/absence can indicate changes in gene expression.
- Use the positive and negative controls to validate your results and ensure the blot was successful.
- Background Noise:
- Check for background noise on the autoradiograph, which may appear as faint smudges or streaks. Minimize background noise by optimizing the washing steps during the procedure.
- Background noise can sometimes interfere with the interpretation of weak signals, so it’s important to distinguish between true signals and noise.
- Data Documentation:
- Document your results by labeling the bands with their corresponding RNA species, sizes, and relative abundances.
- Take photographs or save digital images of the autoradiograph or detection method results for reference and future analysis.
- Data Presentation:
- Organize and present your results in a clear and understandable format, such as a labeled gel image with annotations indicating RNA sizes and abundance.
- Create graphs or figures to illustrate changes in RNA abundance across different samples or experimental conditions if required.
- Statistical Analysis (if applicable):
- If you have multiple replicates or conditions, perform statistical analysis to determine the significance of observed differences in RNA abundance.
- Biological Interpretation:
- Interpret the results in the context of your research objectives. Consider the biological significance of changes in RNA expression and how they relate to your experimental conditions or hypotheses.
- Conclusions and Reporting:
- Draw conclusions based on your data and discuss their implications. Address whether your findings support or contradict your initial hypotheses.
- Report your results in research papers, presentations, or other scientific communications, making sure to include details of the experimental setup and controls.
Troubleshooting and Tips:
- Weak Signal or No Signal:
- Check the probe quality and labeling efficiency.
- Ensure proper hybridization conditions (e.g., temperature, time).
- Confirm that the RNA transferred to the membrane successfully.
- Verify the probe’s specificity and target sequence.
- High Background Noise:
- Optimize the washing steps to reduce background noise.
- Use stringent washing conditions.
- Ensure thorough blocking of the membrane to prevent nonspecific binding.
- RNA Degradation:
- Handle RNA samples and reagents with care to minimize RNase contamination.
- Use RNase-free equipment and labware.
- Store RNA samples at -80°C or in RNase-free conditions.
- Uneven Gel Loading:
- Load RNA samples carefully to avoid overloading or underloading lanes.
- Use an RNA marker to verify gel loading accuracy.
- Incomplete RNA Transfer:
- Ensure proper transfer of RNA from the gel to the membrane.
- Verify that the membrane was appropriately fixed and cross-linked.
- Inconsistent Results:
- Maintain consistent experimental conditions, including buffer concentrations, temperature, and timings.
- Use the same lot of reagents throughout the experiment.
- Probe Contamination:
- Use clean, sterile tubes and pipette tips for probe preparation.
- Avoid repeated freeze-thaw cycles of probe stocks.
- Sample Handling:
- Label tubes and membranes clearly to prevent mix-ups.
- Handle radioactive materials safely and according to laboratory protocols.
- Proper Documentation:
- Keep detailed records of your experiment, including conditions, probe sequences, and exposure times.
- Document each step and any deviations from the protocol.
- Controls and Replicates:
- Always include positive and negative controls.
- Use replicates to ensure the reliability of your results.
- Verify RNA Integrity:
- Assess RNA integrity on a gel before Northern blotting.
- Ensure RNA samples are not degraded or contaminated.
- Optimize Hybridization Conditions:
- Test various hybridization temperatures and times to find the optimal conditions for your probe and RNA targets.
- Use Fresh Solutions:
- Prepare fresh buffers and solutions for each experiment to ensure consistency and reliability.
- Practice Patience:
- Northern blotting can be a finicky technique. Be patient and persistent in optimizing conditions and troubleshooting issues.
- Consult Colleagues and Literature:
- Seek advice from experienced researchers or consult relevant literature for additional troubleshooting tips specific to your experiment.
- Quality Control:
- Always validate your Northern blot results using other RNA analysis techniques like qRT-PCR or RNA-Seq when possible.
Advantages and Disadvantages of Northern Blotting:
| Advantages of Northern Blotting | Disadvantages of Northern Blotting |
|---|---|
| Specific RNA detection | Low sensitivity |
| Quantitative data | Labor-intensive |
| Accurate size determination | Limited throughput |
| Study gene expression patterns | Challenging probe design |
| Analyze non-coding RNAs | Safety concerns (radioactive probes) |
| Customizable for specific targets | Semi-quantitative quantification |
| Validation tool for other methods | Risk of RNA degradation |
| Educational tool | Background noise |
Limitations of Northern Blotting:
- Low Sensitivity: Northern blotting is less sensitive than newer RNA analysis techniques like qRT-PCR and RNA sequencing, making it unsuitable for detecting low-abundance RNA molecules.
- Labor-Intensive: The procedure involves multiple steps and is time-consuming and labor-intensive.
- Limited Throughput: It is not suitable for high-throughput analysis due to its time and resource requirements.
- Challenging Probe Design: Designing and labeling probes can be complex, and probe quality can impact results.
- Radioactive Materials (if using radioactive probes): Handling radioactive probes requires safety precautions and regulatory compliance, which may limit its use in some labs.
- Semi-Quantitative: Northern blotting provides semi-quantitative data at best and may not offer absolute quantification of RNA molecules.
- Risk of RNA Degradation: RNA can degrade during various steps of the procedure if precautions are not taken.
- Background Noise: Background noise can be a common issue that may interfere with result interpretation.
- Limited Resolution: It may not resolve closely related RNA species or isoforms.
- Time-Consuming Controls: Controls and replicates may increase the time and resources required for each experiment.
Variations and Modern Alternatives:
Variations:
- Western Blotting: Similar to Northern blotting but used for detecting specific proteins rather than RNA.
- Southern Blotting: Used for DNA analysis, primarily to detect specific DNA sequences.
- RNase Protection Assay: Measures specific RNA transcripts’ abundance by protecting them from degradation by RNase enzymes.
Modern Alternatives:
- qRT-PCR (Quantitative Reverse Transcription Polymerase Chain Reaction): Provides highly sensitive and quantitative RNA analysis, suitable for high-throughput studies.
- RNA Sequencing (RNA-Seq): High-throughput method that offers comprehensive analysis of the entire transcriptome, including RNA quantification and identification of novel transcripts.
- Microarray Analysis: Allows the simultaneous measurement of expression levels for thousands of genes or transcripts.
- Digital PCR: Provides absolute quantification of RNA molecules in a sample, even at low abundance.
- Next-Generation Sequencing (NGS): Includes RNA-Seq as well as other sequencing techniques for studying RNA molecules, offering high-throughput and in-depth insights.
- In Situ Hybridization (ISH): Visualizes RNA molecules within cells or tissues, providing spatial information on gene expression.
- NanoString Technology: Enables the direct measurement of RNA abundance in a high-throughput and digital manner.
- Droplet Digital PCR (ddPCR): Similar to digital PCR, this method provides absolute quantification of RNA molecules in individual droplets.
- CRISPR/Cas9-based Techniques: Methods like CRISPR-Cas9 knockdown or knockout can be used to study the functional consequences of gene expression changes.
Comparison of Northern Blotting with Modern Techniques:
| Aspect | Northern Blotting | qRT-PCR | RNA-Seq |
|---|---|---|---|
| Sensitivity | Low (may not detect low-abundance RNA) | High (detects low-abundance RNA) | High (detects low-abundance RNA) |
| Quantification | Semi-quantitative | Quantitative | Quantitative |
| Throughput | Low (not suitable for high-throughput) | Medium (medium-throughput) | High (high-throughput) |
| Resolution | Limited (may not resolve closely related RNA species) | Good | Excellent |
| Customization | Customizable for specific targets | Customizable | Broad applicability |
| Probe Design | Challenging | Relatively straightforward | Not applicable (sequence-based) |
| Data Depth | Limited | Specific gene or target | Comprehensive (whole transcriptome) |
| Time and Labor | Time-consuming and labor-intensive | Moderate time and labor | High-throughput, automated |
| Controls | Required (positive and negative controls) | Required (positive and negative controls) | Required (quality control) |
| Safety Concerns | Radioactive probes (if used) | None | None |
| Cost | Moderate | Moderate | High |
| Applications | Specific RNA detection, gene expression analysis | Gene expression, validation | Comprehensive RNA analysis |
FAQs:
Q1: What is Northern blotting used for?
Northern blotting is used to detect and study RNA molecules in a sample. It is commonly used for gene expression analysis, RNA size determination, and the detection of specific RNA transcripts.
Q2: How does Northern blotting differ from Southern blotting?
Northern blotting is used to study RNA, while Southern blotting is used to study DNA. Both techniques involve electrophoresis, transfer to a membrane, and hybridization with a probe, but they target different nucleic acids.
Q3: What is the main advantage of Northern blotting?
The main advantage of Northern blotting is its ability to provide specific information about RNA molecules, including their size and abundance. It also allows researchers to study gene expression patterns.
Q4: What are the limitations of Northern blotting?
Some limitations of Northern blotting include low sensitivity for low-abundance RNA, labor-intensiveness, limited throughput, and the need for careful handling of radioactive probes (if used).
Q5: How do I choose between Northern blotting and other RNA analysis methods like qRT-PCR or RNA-Seq?
The choice depends on your research goals. Northern blotting is suitable for specific RNA detection and size determination. qRT-PCR offers high sensitivity and quantitative data. RNA-Seq provides comprehensive transcriptome analysis.
Q6: Is Northern blotting still widely used in modern research?
While Northern blotting has been partially replaced by newer, high-throughput methods, it is still used in research where specific RNA detection and size determination are required.
Q7: What are the key steps in a Northern blotting procedure?
Key steps in Northern blotting include RNA extraction, gel electrophoresis, membrane transfer, probe hybridization, washing, detection, and result interpretation.
Q8: Can I use non-radioactive probes for Northern blotting?
Yes, non-radioactive probes, such as enzyme-labeled or fluorescent probes, can be used as alternatives to radioactive probes in Northern blotting.
Q9: What safety precautions are needed for working with radioactive probes in Northern blotting?
When using radioactive probes, follow laboratory safety protocols, use appropriate shielding, and dispose of radioactive waste properly. Researchers should be trained in handling radioactive materials.
Q10: Can Northern blotting be used for clinical diagnostics?
Yes, Northern blotting has been used in clinical settings to diagnose certain genetic disorders characterized by abnormal RNA patterns. However, it may be less common than other diagnostic methods today.
Conclusion:
In conclusion, Northern blotting is a valuable molecular biology technique that allows for the specific detection, size determination, and gene expression analysis of RNA molecules. Despite its limitations, such as lower sensitivity and labor-intensiveness, it remains a valuable tool in certain research contexts. Researchers often choose Northern blotting when they require specific RNA detection and size information, and it can complement other modern RNA analysis techniques like qRT-PCR and RNA sequencing.
As technology advances, researchers now have access to a range of alternative methods that offer higher sensitivity, throughput, and quantification capabilities. The choice of RNA analysis method should be based on the specific research goals, available resources, and the need for sensitivity, quantification, throughput, and resolution.
Home | Blog | About Us | Contact Us | Disclaimer
Possible References Used





2 Comments