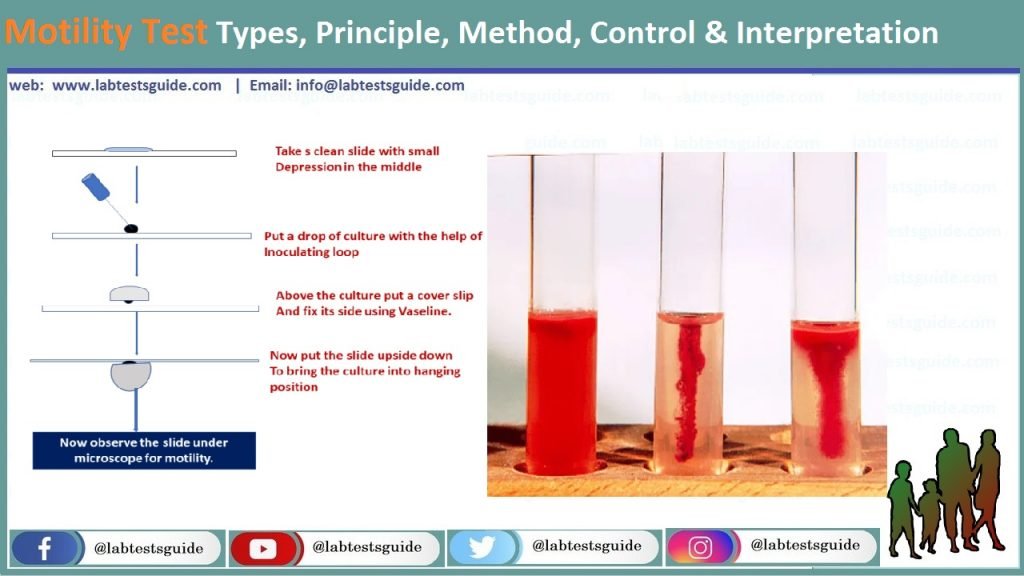
Motility test can define as the analytical method, which examines whether the bacteria are motile or non-motile hrough biochemical and microscopic analysis. Tube test is a biochemical method giving macroscopic and cumulative results obtained by the reduction of the biological media (triphenyl tetrazolium chloride) by the positive or motile organisms. Slide test is a microscopic analysis of a microorganism that involves direct examination through hanging drop and wet mount slide test, based on the cell’s microscopic features.
Purpose
- Motility test aims to check the cellular motility of a test organism.
- It provides a valuable tool to determine an organism’s ability to locomote within the liquid or semi-solid media.
- Motility test also distinguishes different kinds of bacteria.
Example: Like Yersinia enterocolitica tends to be motile at a temperature of 20-25 degrees Celsius. Capnocytophyta is the bacteria that move via gliding motility, whereas few bacteria like Campylobacter species are non-motile.
Two methods are gives:
- Wet Mount
- The Tube Motility Test
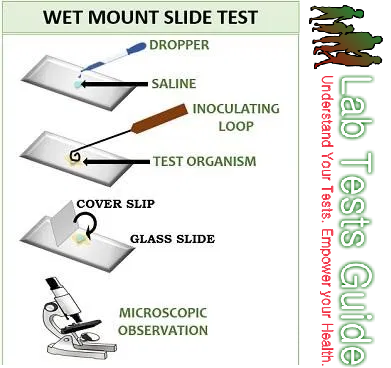
1. WET MOUNT PROCEDURE
Procedure :
- Make suspension of a colony of test organisms in distilled water on a glass slide. Alternatively, a loop of medium from a fresh broth culture can be used. Put a cover glass on it. Examine under the microscope using hypochlorite solution as it contains live organisms.
- Hanging Drop Method: Clean a cover slip. Apply Vaseline at its 4 corners. Put a drop of distilled water in the center and emulsify a colony of test organisms. Put the glass slide gently on it and hold it up side down. See under microscope with 10X and then 40X objective. Margins of drops are specifically seen. Motile organisms are clearly seen moving rapidly in the field. Non-motile organisms show to-and-fro Brownian motion, but these don’t move in relation to each other.
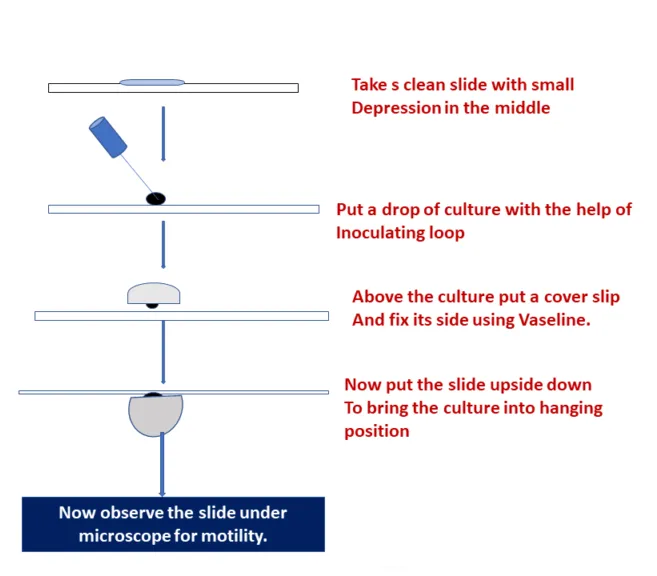
2. TUBE MOTILITY TEST
Procedure:
- Using the sterile inoculating needle, remove suspicious colony of an 18-24 hour culture. Inoculate motility agar medium (Peptone water with 0.2% New Zealand agar, semisolid agar) carefully stabbing the needle 3-4 cm into the medium then withdrawing the needle so that a line of inoculum can be seen.
- Incubate the tube aerobically at 35-37°C for 18-24 hour.
- Tetrazolium salts may be added to motility media to make them easier to read.
- The salt is colorless, but as the organisms grow, the dye gets incorporated into the bacterial cells, where it is reduced to an insoluble red pigment.
Interpretation:
Non-motile organisms, such as B. anthracis, from a single line of growth that does not deviate from the original inoculum stab. Motile organisms from a diffuse growth zone around the inoculum stab.
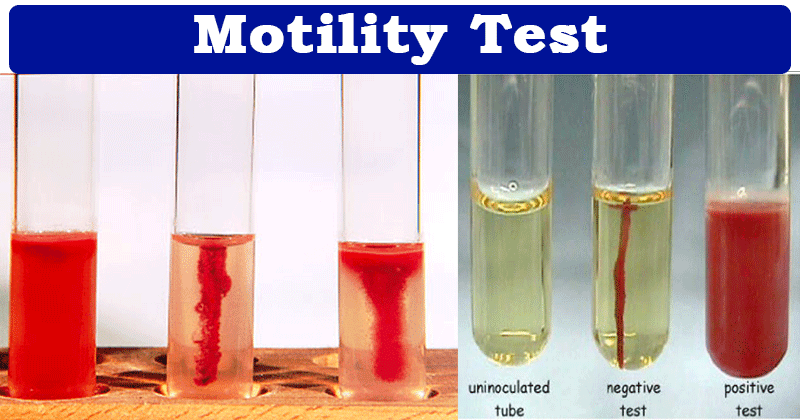
Control:
Pseudomonas aeruginosa ATCC 35032 or equivalent is non-motile.
Method:
- Control strains should be assayed on each day of testing.
- Resolving an out-of-control result needs checking the purity, and identity of the control strains and repeating the test.
- Tetrasolium salts may be added to motility medium to make them easier to read.
- The salt is colorless but as the organism grows, the dye is incorporated into the bacterial cells where it is reduced to an insoluble red pigment formazan.
Uses
Motility test distinct microorganisms as motile or non-motile, based on their motility. It also helps in the taxonomic classification of bacteria focussing on macroscopic and microscopic characteristics.
Motility test aids in characterization or identification of pathogens or non-pathogens (as motility is one of virulence factor), and also facilitates species-level differentiation.
Example: Distinguishes between Enterococcus faecalis (non-motile) and Enterococcus gallinarum (motile).
Limitations
In tube motility test, the inoculating needle should be taken out along the same line of inoculation otherwise, it may give false-positive growth. The water condensation in the tube motility test may lead to the false-positive results, and weakly motile organisms may give contrast result interpretation.
Related Articles:
Possible References Used