The Mantoux tuberculin skin test, also known as the PPD (purified protein derivative) test, is a diagnostic tool used to determine if a person has been exposed to the bacteria that cause tuberculosis (TB). The test involves injecting a small amount of a substance called tuberculin into the skin of the forearm. Tuberculin is derived from the tuberculosis bacteria and contains proteins that can elicit an immune response in individuals who have been exposed to TB.
| Also Known as | Tuberculin Skin Test (TST), Purified Protein Derivative (PPD), Latent Tuberculosis Infection Test, Mantoux Tuberculin Skin Test, Tuberculosis Skin Test (tst), TB skin test, Mantoux Test |
| Test Purpose | A TB skin test is used to screen for tuberculosis infection when someone has potentially been exposed to tuberculosis. |
| Test Preparations | No Sample Preparation Needed |
| Test Components | Mantoux Test |
| Specimen | No Need |
| Stability Room | n/a |
| Stability Refrigerated | — |
| Stability Frozen | — |
| Method | — |
| Download Report | Download Report |
Principle:
- PPD (purified protein derivative) is subcutaneously injected to the patient and individual’s sensitivity to tuberculin protein is assessed. If the patient already had a previous exposure to M. tuberculosis, a hypersensitivity reaction takes place.
- The antigen produces induration (thickening) at the site of injection. The diameter of the induration is measured to evaluate the test and is directly proportional to the level of sensitization. American Thoracic Society (ATS) has recommended 5 Tuberculin Units (0.1 ml) as a standard regimen for the Mantoux Test.
- In highly endemic areas, this standard dose could be reduced as per the guidelines.
Requirements:
- Graduated syringe (of 1 ml) with a short bevel needle (26 G)
- Tuberculin PPD (Mantoux solution)
- Spirit swab
Procedure:
- Bring the tuberculin PPD to room temperature.
- Draw up just over 0.1ml of tuberculin protein into a graduated syringe.
- Remove air and excess tuberculin to leave exactly 0.1ml of tuberculin.
- The ideal site is dorsal surface of the forearm (usually left) about 4 cm below the elbow joint.
- Clean the site with spirit swab and allow to dry.
- Insert the needle parallel with the skin surface, advance the bevel 3-5 mm and inject the PPD.
- Remove the needle without pressing the injection site.
- Wait for 48-72 hours and observe the induration. The area of erythema is not included.
Reading the TB skin test:
“Reading” the skin test means detecting a raised, thickened local area of skin reaction, referred to as induration. Induration is the key item to detect, not redness or bruising. Read skin tests 48-72 hours after the injection when the size of the induration is maximal. Tests read after 72 hours tend to underestimate the size of the induration and are not accurate
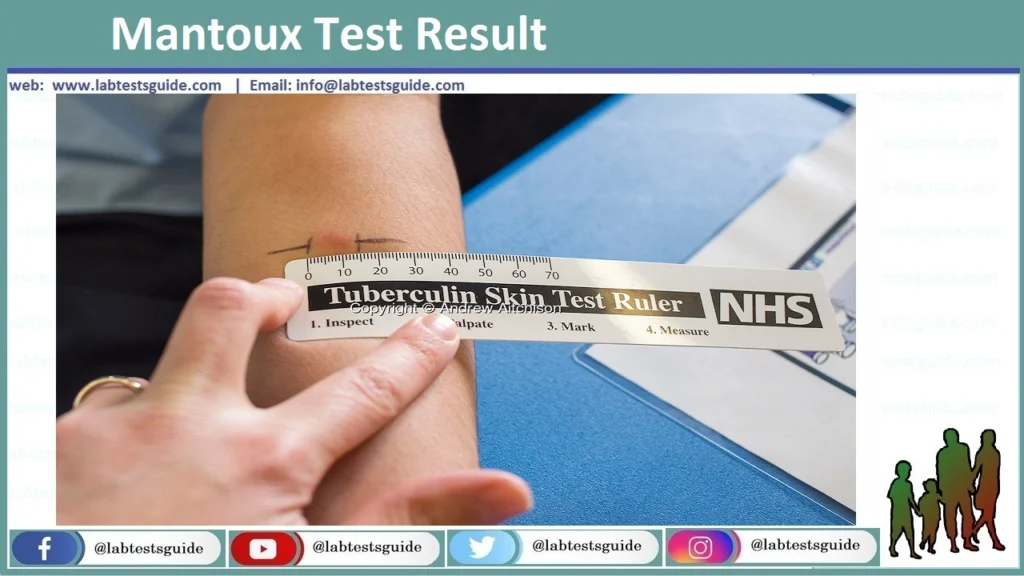
- The negative case when there is no induration.
- In positive cases, there will be induration at the site of injection appear in 48 to 72 hours.
- Induration < 5 mm D is seen in:
- HIV patients or immunodeficiency state.
- Close contact with active TB case.
- The patient with X-Ray finding of old TB.
- Induration > 10 mm in D seen in:
- IV drug users.
- Nursing home residents.
- Traveler from high TB areas.
- A worker in the home care facility.
- Malnutrition, postgastrectomy, steroid use and Diabetes.
- Induration > 15 mm in diameter seen in:
- Patient with active TB.
- All patients who don’t fulfill the above criteria.
The positive test is seen in:
- An active case of TB.
- Other types of Mycobacterial infection.
The negative test is seen in:
- Immune incompetent chronically ill patient.
- Patient not exposed to TB.
INTERPRETATION OF MANTOUX TEST:
- A positive Mantoux test indicates a previous exposure to M. tuberculosis but not necessarily an active disease.
- The Mantoux test causes a booster effect to produce a positive result in an individual who has been already exposed previously.
- The Mantoux test usually becomes positive 4-6 weeks after infection.
- If a papule does not appear after the injection, the test should be repeated on other arm as the solution has been injected too deeply.
- False negative results are seen if there is bacterial contamination of the tuberculin solution, corticosteroid therapy, viral infections (HIV, influenza, EBV) and poor nutrition.
- False positive results are seen in individuals vaccinated with BCG (reactions are 5-10 mm diameter).
- If the induration is 15 mm or more in BCG vaccinated individual, it should be considered positive.
Possible References Used