Ion selective electrode (ISE) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. ISE has many advantages compared to other techniques, including:
- It is relatively inexpensive and easy to operate.
- It has wide concentration measurement range.
- As it measure the activity, instead of concentration, it is particularly useful in biological/medical application.
- It is a real-time measurement, which means it can monitor the change of activity of ion with time.
- It can determine both positively and negatively charged ions.
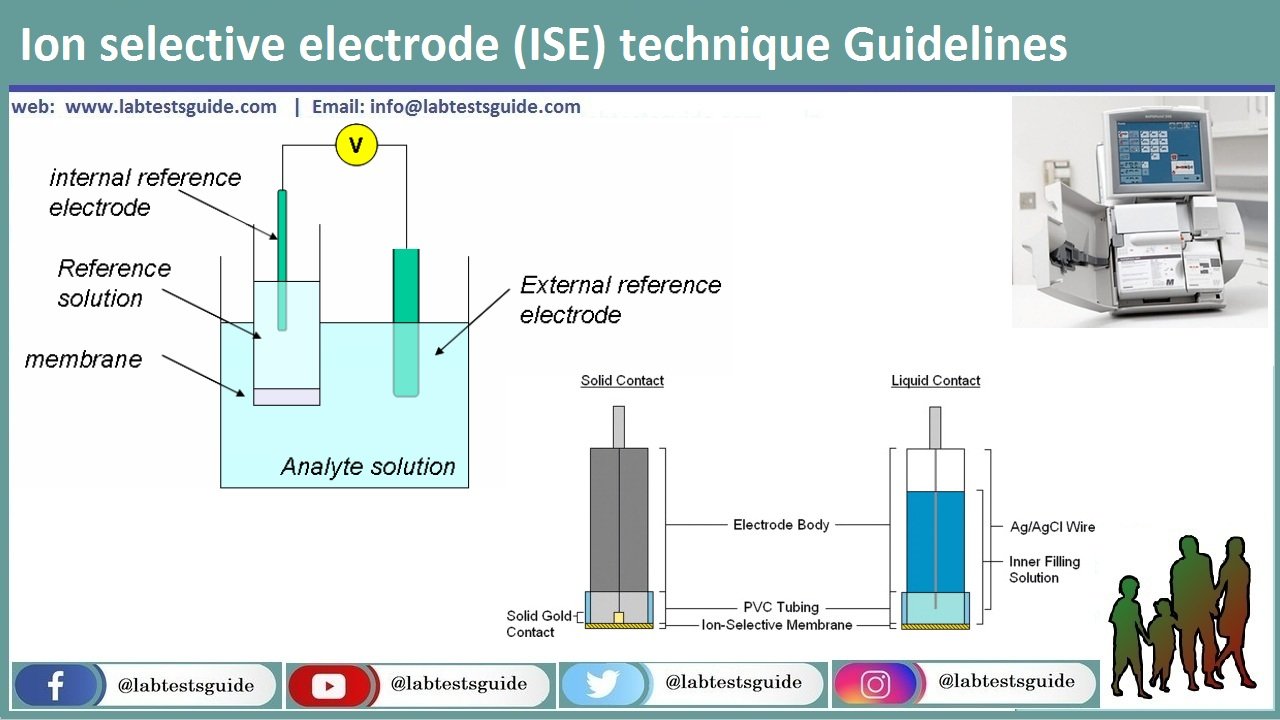
Based on these advantages, ISE has wide variety of applications, which is reasonable considering the importance of measuring ion activity. For example, ISE finds its use in pollution monitoring in natural waters (CN-, F-, S-, Cl-, etc.), food processing (NO3-, NO2- in meat preservatives), Ca2+ in dairy products, and K+ in fruit juices, etc.
Types:
- Glass membranes
- Crystalline membranes
- Ion-exchange resin membranes
- Enzyme electrodes
1. Glass membranes:
Glass membranes are made of a type of ion exchange glass (silicate or chalcogenide). This type of ISE has good selectivity, but only for various single-charged cations; mainly H +, Na + and Ag +. Chalcogenide glass also has selectivity for double-charged metal ions, such as Pb2 + and Cd2 +. The glass membrane has excellent chemical durability and can work in very aggressive media. A very common example of this type of electrode is the pH glass electrode.
2. Crystalline membranes:
Crystalline membranes are made of mono or polycrystallites of a single substance. They have good selectivity, because only ions that can get into the crystal structure can interfere with the response of the electrode. This is the main difference between this type of electrode and glass membrane electrodes. The lack of internal solution reduces possible unions. The selectivity of crystalline membranes can be for both the cation and the anion of the membrane-forming substance. An example is the LaF3 crystal-based fluoride selective electrode.
3. Ion-exchange resin membranes
Ion exchange resins are based on special organic polymer membranes that contain a specific ion exchange substance (resin). This is the most widespread type of ion specific electrode. The use of specific resins allows the preparation of selective electrodes for dozens of different ions, both single and multi-atom. They are also the most widespread electrodes with anion selectivity. However, such electrodes have low chemical and physical durability, as well as a “survival time”. An example is the potassium selective electrode, based on valinomycin as an ion exchange agent.
4. Enzyme electrodes
Enzyme electrodes are definitely not true ion selective electrodes, but they are generally considered within the topic of ion specific electrodes. This electrode has a “double reaction” mechanism: an enzyme reacts with a specific substance and the product of this reaction (usually H + or OH-) is detected by a true ion-selective electrode, such as pH-selective electrodes. All of these reactions occur within a special membrane that covers the true ion-selective electrode, which is why enzymatic electrodes are sometimes considered ion-selective. An example is glucose selective electrodes.
Advantages and limitations of I.S.E.
Advantages:
- Linear response: over 4 to 6 orders of magnitude of A.
- Non-destructive: no consumption of analyte.
- Non-contaminating.
- Short response time: in sec. or min. useful in indust. applications.
- Unaffected by color or turbidity.
Limitations:
- Precision is rarely better than 1%.
- Electrodes can be fouled by proteins or other organic solutes.
- Interference by other ions.
- Electrodes are fragile and have limited shelf life.
- Electrodes respond to the activity of uncomplexed ion. So
ligands must be absent or masked. m must be kept constant
Related Articles:
Possible References Used