Iron-Hematoxylin Staining 50 FAQs and 30 MCQS:
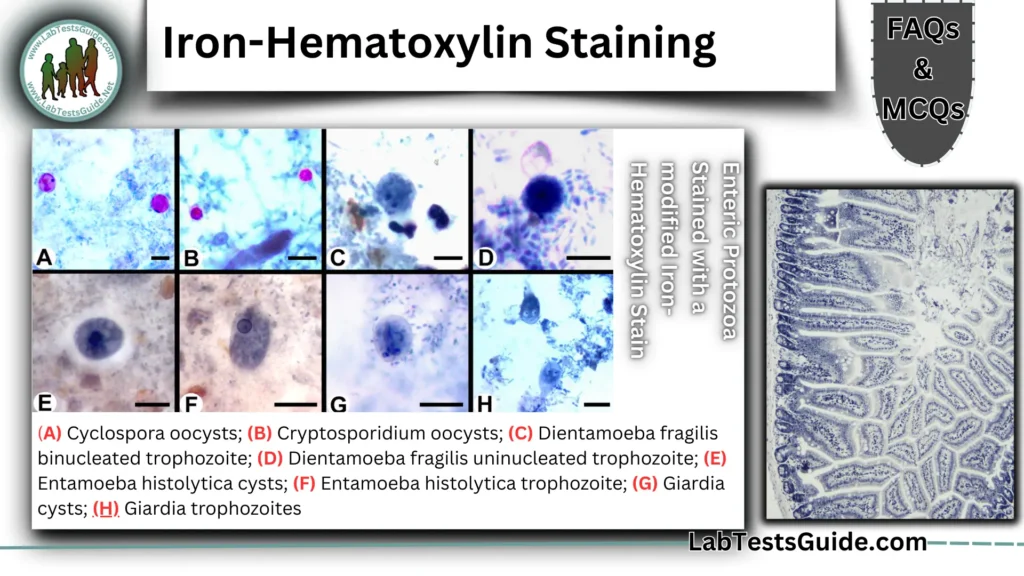
Iron-Hematoxylin Staining 50 FAQs
What is Iron-Hematoxylin staining used for?
It detects intestinal protozoans by staining nuclear structures for clear microscopic identification.
Why is Iron-Hematoxylin preferred for parasite diagnosis?
It provides permanent, high-clarity stains for protozoan morphology.
What specimens can be used with Iron-Hematoxylin staining?
Fresh, SAF-preserved, or PVA-preserved stool samples.
What is the principle behind Iron-Hematoxylin staining?
Hematoxylin oxidizes to hematein, binds with ferric ammonium sulfate (mordant), and forms a stable dye for nuclear staining.
What is the role of ferric ammonium sulfate in the stain?
It acts as a mordant to form the iron-hematoxylin complex for sharp staining.
How is hematoxylin converted into an effective dye?
It undergoes oxidation to form hematein, the active dye component.
What happens if hematoxylin is overoxidized?
It forms oxyhematein, which is non-staining.
How long can Iron-Hematoxylin working solution be stored?
Up to 7 days (filter periodically to remove debris)
What is Schaudinn’s solution, and why is it used?
A fixative containing mercuric chloride, ethanol, and acetic acid; preserves cell structures.
How do you prepare the iodine-alcohol solution?
Mix 98 mL of 95% ethanol with 2 mL tincture of iodine.
What is the purpose of picric acid in the staining process?
It differentiates (destains) excess dye for clearer nuclear visibility.
Why is hydrochloric acid added to the mordant solution?
Enhances staining precision by controlling pH.
Can you substitute ferric chloride for ferric ammonium sulfate?
Yes (e.g., in Heidenhain’s variant), but concentrations vary.
How do you test if the working stain is still active?
Add drops to alkaline tap water: blue = good; brown = replace.
How long should smears fix in Schaudinn’s solution?
Minimum 30 minutes.
Why is mercuric chloride removed before staining?
Residual mercury interferes with staining and causes refractive artifacts.
How is PVA-preserved stool prepared for staining?
Mix with PVA, dry on slides (37°C incubator or overnight at RT).
What’s the purpose of the 70% ethanol wash post-fixation?
Removes excess Schaudinn’s fixative.
Why is iodine-alcohol used after fixation?
Removes mercury crystals from Schaudinn’s/PVA-fixed smears.
How long should slides stay in hematoxylin working solution?
4–10 minutes (varies by protocol).
Why is tap water rinsing critical after staining?
Stops the reaction and removes excess dye.
What’s the role of xylene in the process?
Clears and dehydrates slides before mounting.
How do you differentiate SAF-preserved samples?
Use Mayer’s albumin for smear adhesion, then follow SAF protocol.
Can Iron-Hematoxylin stain helminth eggs?
No—best for protozoans; helminths need wet mounts.
Why do nuclei appear faint or unstained?
Under-oxidized hematoxylin, insufficient mordanting, or over-differentiation.
What causes excessive background staining?
Inadequate washing or incomplete mercury removal.
How to fix refractive granules in stained slides?
Replace iodine-alcohol to fully remove mercuric chloride.
Why do slides look brown instead of blue-black?
Old/overoxidized stain or incorrect pH during washing.
What if the smear detaches during staining?
Insufficient drying or improper albumin adhesion (for SAF).
How should protozoan nuclei appear after staining?
Dark purple/black with pale cytoplasm.
What color do RBCs stain with Iron-Hematoxylin?
Pale yellow/green.
How do chromatoid bodies appear?
Dark blue/black rods or granules.
Can Iron-Hematoxylin identify Cyclospora or Cryptosporidium?
Yes (requires modified protocols with carbol fuchsin).
What’s the difference between Heidenhain’s and Weigert’s Iron-Hematoxylin?
Heidenhain’s uses ferric ammonium sulfate; Weigert’s uses ferric chloride.
How does the Scholten modification differ?
Uses picric acid for destaining and skips acid alcohol.
Can you counterstain Iron-Hematoxylin slides?
Yes (e.g., eosin), but nuclei should remain the focal point.
How should Schaudinn’s solution be disposed of?
As hazardous waste (contains mercury).
What’s the shelf life of stock hematoxylin solution?
12 months at room temperature (protected from light).
Why must working solution be filtered periodically?
Prevents debris from affecting stain quality.
Can Iron-Hematoxylin stain Dientamoeba fragilis?
Yes—shows binucleated trophozoites clearly.
Is it useful for Giardia cysts?
Yes—stains nuclei and axonemes.
Why is it not ideal for Isospora belli?
Oocysts are better visualized with acid-fast stains.
How to perform QC for Iron-Hematoxylin stain?
Test with known positive protozoan samples.
What indicates a failed QC slide?
Poor nuclear contrast or excessive background.
How does Iron-Hematoxylin compare to Trichrome staining?
Iron-Hematoxylin is more permanent; trichrome is faster but less durable.
Which is better for archival slides: Iron-Hematoxylin or Ziehl-Neelsen?
Iron-Hematoxylin (long-term stability).
Can you automate Iron-Hematoxylin staining?
Manual protocols are standard for precision.
Why avoid over-drying PVA smears?
Causes cracking and poor stain penetration.
What’s the purpose of permount?
Preserves slides under coverslips for long-term storage.
Can Iron-Hematoxylin stain bacterial cells in specimens?
Yes—they appear dark blue/black but aren’t diagnostic targets.
Iron-Hematoxylin Staining 30 MCQS:
- What is the primary purpose of Iron-Hematoxylin staining?
a) Staining bacterial cell walls
b) Detecting intestinal protozoan nuclei✔
c) Identifying fungal hyphae
d) Highlighting collagen fibers - Which component acts as the mordant in Iron-Hematoxylin staining?
a) Hematoxylin
b) Ferric ammonium sulfate✔
c) Hydrochloric acid
d) Ethanol - What is the active dye formed when hematoxylin oxidizes?
a) Oxyhematein
b) Hematein✔
c) Eosin
d) Carbol fuchsin - Why must Schaudinn’s fixative be thoroughly washed off before staining?
a) It inhibits hematein formation
b) It contains mercury, which interferes with staining✔
c) It dissolves nuclear material
d) It causes over-oxidation - Which solution removes mercuric chloride from fixed smears?
a) Xylene
b) Iodine-alcohol✔
c) Picric acid
d) Permount
- How long should slides remain in Schaudinn’s fixative for optimal results?
a) 5 minutes
b) 30 minutes✔
c) 2 hours
d) Overnight - What is the purpose of the 70% ethanol wash after fixation?
a) To dehydrate the smear
b) To remove excess fixative✔
c) To oxidize hematoxylin
d) To counterstain - Which step follows the hematoxylin staining in the protocol?
a) Differentiation with picric acid
b) Mounting with permount
c) Rinsing with tap water✔
d) Clearing with xylene - Why is picric acid used in some Iron-Hematoxylin protocols?
a) To fix the specimen
b) To differentiate (destain) excess dye✔
c) To oxidize hematoxylin
d) To preserve slides - How long can the Iron-Hematoxylin working solution be stored?
a) 1 day
b) 1 week✔
c) 1 month
d) 1 year
- Which preservative is compatible with Iron-Hematoxylin staining?
a) Formalin
b) PVA (Polyvinyl-alcohol)✔
c) Bouin’s solution
d) Glutaraldehyde - How should PVA-preserved stool samples be prepared for staining?
a) Centrifuge and use supernatant
b) Mix with PVA, dry on slides, and fix✔
c) Directly stain without fixation
d) Freeze before use - What is the purpose of Mayer’s albumin in SAF-preserved samples?
a) To act as a mordant
b) To adhere sediment to slides✔
c) To oxidize hematoxylin
d) To remove mercury - Which step is unique to SAF-preserved specimens?
a) Post-fixation with Schaudinn’s
b) Use of iodine-alcohol
c) Centrifugation to collect sediment✔
d) Staining with carbol fuchsin - Why must PVA smears be dried thoroughly before staining?
a) To prevent cracking✔
b) To enhance hemoglobin staining
c) To avoid over-oxidation
d) To reduce background debris
- How should protozoan nuclei appear after proper staining?
a) Pale yellow
b) Dark purple/black✔
c) Bright red
d) Green-blue - What does faint nuclear staining indicate?
a) Over-oxidation of hematoxylin
b) Insufficient mordanting
c) Excessive picric acid
d) All of the above✔ - What causes refractive granules in stained slides?
a) Incomplete mercury removal✔
b) Over-staining
c) Insufficient drying
d) Contaminated xylene - Which organism is NOT well-visualized with Iron-Hematoxylin?
a) Entamoeba histolytica
b) Giardia lamblia
c) Ascaris lumbricoides (helminth egg)✔
d) Dientamoeba fragilis - How to test if the working stain is still active?
a) Mix with saline—look for precipitation
b) Add to alkaline tap water—blue = good✔
c) Heat to 60°C—check for color change
d) Expose to UV light—fluorescence indicates viability
- Which modification uses ferric chloride instead of ferric ammonium sulfate?
a) Heidenhain’s
b) Weigert’s✔
c) Scholten’s
d) Kinyoun’s - What is the key advantage of Iron-Hematoxylin over Trichrome stain?
a) Faster procedure
b) Permanent slides✔
c) Better for helminths
d) No need for fixation - Which step is omitted in the Scholten modification?
a) Iodine-alcohol
b) Acid alcohol differentiation✔
c) Xylene clearing
d) Tap water rinse - Why is Iron-Hematoxylin unsuitable for Isospora belli?
a) Oocysts stain poorly
b) Requires acid-fast stains✔
c) Over-stains cytoplasm
d) Destroys oocyst walls - Which counterstain pairs well with Iron-Hematoxylin?
a) Methylene blue
b) Eosin✔
c) Safranin
d) Crystal violet
- How should Schaudinn’s fixative waste be disposed of?
a) Down the sink
b) As hazardous waste (mercury content)✔
c) Autoclave and landfill
d) Incineration - What is the shelf life of stock hematoxylin solution?
a) 1 month
b) 6 months
c) 12 months✔
d) Indefinite - Why must staining dishes be covered?
a) To prevent evaporation
b) To avoid light exposure
c) To reduce contamination
d) All of the above✔ - Which component is NOT in the mordant solution?
a) Ferric ammonium sulfate
b) Hydrochloric acid
c) Hematoxylin✔
d) Distilled water - What indicates a failed QC slide?
a) Blue tap water test
b) Poor nuclear contrast✔c) Rapid staining time
d) Faded background
Possible References Used







