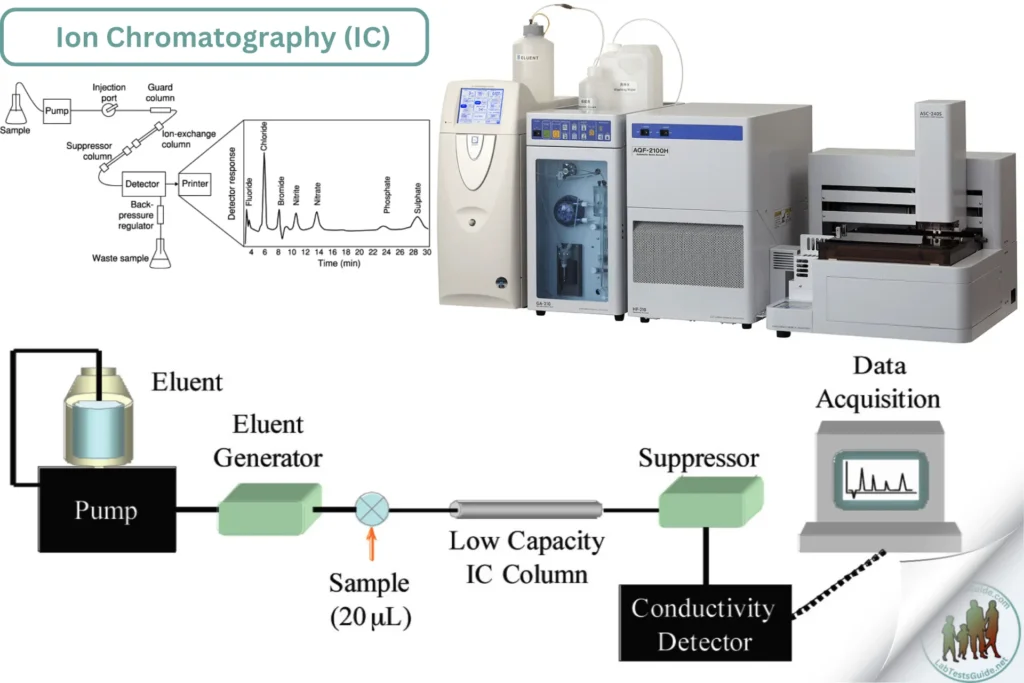
Ion Chromatography (IC) is a powerful analytical technique used to separate and quantify ions in various samples. In clinical laboratories, IC is employed for analyzing biological fluids, determining electrolyte levels, and detecting trace amounts of toxic ions. IC is particularly useful for measuring anions (such as chloride, nitrate, and sulfate) and cations (such as sodium, potassium, and calcium).
Methodology for Ion Chromatography (IC) in Clinical Laboratory
Introduction
- Ion Chromatography (IC) is a powerful analytical technique used to separate and quantify ions in various samples. In clinical laboratories, IC is employed for analyzing biological fluids, determining electrolyte levels, and detecting trace amounts of toxic ions. IC is particularly useful for measuring anions (such as chloride, nitrate, and sulfate) and cations (such as sodium, potassium, and calcium).
Principle
- IC separates ions based on their interaction with a resin column under the influence of an eluent. Ions are detected and quantified as they elute from the column at different retention times. Detection is usually accomplished using a conductivity detector, although other detectors such as UV-Vis or amperometric detectors can also be used.
Specimen Requirements
- Type of specimen: Blood, urine, plasma, serum, or other biological fluids
- Volume of specimen required: Typically 0.5-5 mL
- Collection method: Standard clinical procedures for sample collection (e.g., venipuncture, urine collection)
- Handling and storage requirements: Store samples at 4°C for short-term storage or -20°C for long-term storage
- Stability of the specimen: Analytes in biological fluids are generally stable if stored properly; avoid repeated freeze-thaw cycles
Reagents and Materials
- Eluent solutions (e.g., sodium bicarbonate, sodium carbonate)
- Regenerant solutions (if using suppressor columns)
- Calibration standards for target ions
- IC grade water (deionized water)
- Filters (0.22 µm or 0.45 µm) for sample preparation
- Sample vials and caps
- Control samples with known ion concentrations
Equipment
- Ion Chromatograph system (including pump, injector, column, detector, and suppressor if needed)
- Conductivity detector or other appropriate detectors
- Analytical balance
- pH meter (for eluent preparation)
- Microcentrifuge or syringe filters for sample preparation
- Computer with IC data analysis software
Procedure
Sample Preparation:
- Collect biological samples following standard clinical procedures.
- Centrifuge samples at 3000 rpm for 10 minutes to remove particulates (for blood or plasma samples).
- Filter the supernatant using a 0.22 µm or 0.45 µm filter to obtain a clear solution.
- Dilute the filtered sample with IC grade water if necessary to bring the analyte concentration within the calibration range.
Eluent Preparation:
- Prepare the eluent solution according to the manufacturer’s recommendations or specific method requirements.
- Degas the eluent solution using an ultrasonic bath or by sparging with helium to remove dissolved gases.
Instrument Setup:
- Set up the IC system, including the pump, injector, column, detector, and suppressor (if applicable).
- Prime the pump and column with the eluent solution.
- Equilibrate the column with the eluent at the appropriate flow rate until a stable baseline is achieved.
Calibration and Standardization:
- Prepare calibration standards with known concentrations of target ions.
- Inject calibration standards into the IC system and generate a calibration curve by plotting peak area or height versus concentration.
- Use the calibration curve to quantify ions in the samples.
Sample Analysis:
- Inject prepared samples into the IC system.
- Record chromatograms and identify peaks based on retention times.
- Quantify the ions using the calibration curve.
Data Analysis:
- Analyze the chromatograms using IC data analysis software.
- Integrate peak areas or heights and compare them with the calibration curve to determine ion concentrations.
- Report the results, including any necessary calculations and conversions.
Quality Control
- Calibration: Regularly calibrate the IC system using standard solutions.
- Control Samples: Run control samples with known concentrations of ions to ensure accuracy and precision.
- Blanks: Use blanks to check for contamination and background noise.
- System Suitability: Ensure system suitability by checking parameters such as resolution, peak shape, and retention time stability.
Calculation and Interpretation
- Quantification: Use the calibration curve to calculate the concentrations of ions in the samples.
- Result Interpretation: Compare the results with established reference ranges or clinical guidelines for accurate interpretation.
- Statistical Analysis: Perform statistical analysis to ensure the reliability and reproducibility of the results.
Limitations
- Matrix Effects: Biological samples can contain substances that interfere with ion separation and detection.
- Column Fouling: Protein or particulate matter can foul the column, requiring regular maintenance and cleaning.
- Detection Limits: Sensitivity may be limited for very low concentrations of certain ions.
Safety Precautions
- PPE: Use appropriate PPE (gloves, lab coat, safety goggles) when handling samples and reagents.
- Chemical Handling: Handle chemicals and reagents according to safety guidelines and Material Safety Data Sheets (MSDS).
- Waste Disposal: Dispose of waste according to local regulations and institutional guidelines.
References
- Fritz, J. S., & Gjerde, D. T. (2009). Ion Chromatography (3rd ed.). Wiley.
- Clinical Chemistry: Principles, Techniques, and Correlations by Michael L. Bishop, Edward P. Fody, and Larry E. Schoeff.
- Manufacturer’s guidelines for IC systems and reagents.
- Clinical guidelines and standards from institutions such as the American Society for Clinical Pathology (ASCP) and the Clinical and Laboratory Standards Institute (CLSI).
This format provides a structured approach to conducting IC in clinical laboratories, focusing on accurate quantification of ions, quality control, and safety precautions for reliable results.
Possible References Used