HPLC stands for High-Performance Liquid Chromatography, and it is a widely used analytical technique in chemistry and biochemistry for separating, identifying, and quantifying components in a mixture. HPLC is based on the principles of chromatography, which is a method for separating mixtures into their individual components based on differences in their interactions with a stationary phase and a mobile phase.

Introduction:
High-Performance Liquid Chromatography, commonly known as HPLC, is a sophisticated and indispensable analytical technique in the world of chemistry and biology. It plays a crucial role in separating, identifying, and quantifying components within complex mixtures, making it an invaluable tool for researchers, scientists, and analysts across various industries.
HPLC owes its prominence to its ability to provide precise and reliable results. It achieves this by capitalizing on the principles of chromatography, where a sample mixture is separated into its individual constituents based on their differing affinities for a stationary phase and a mobile phase. This separation process is both efficient and highly controllable, making it suitable for a wide range of applications.
Principles of HPLC:
High-Performance Liquid Chromatography (HPLC) is rooted in a set of fundamental principles that drive its effectiveness in separating and analyzing complex mixtures. This section will delve into the core principles that underpin HPLC:
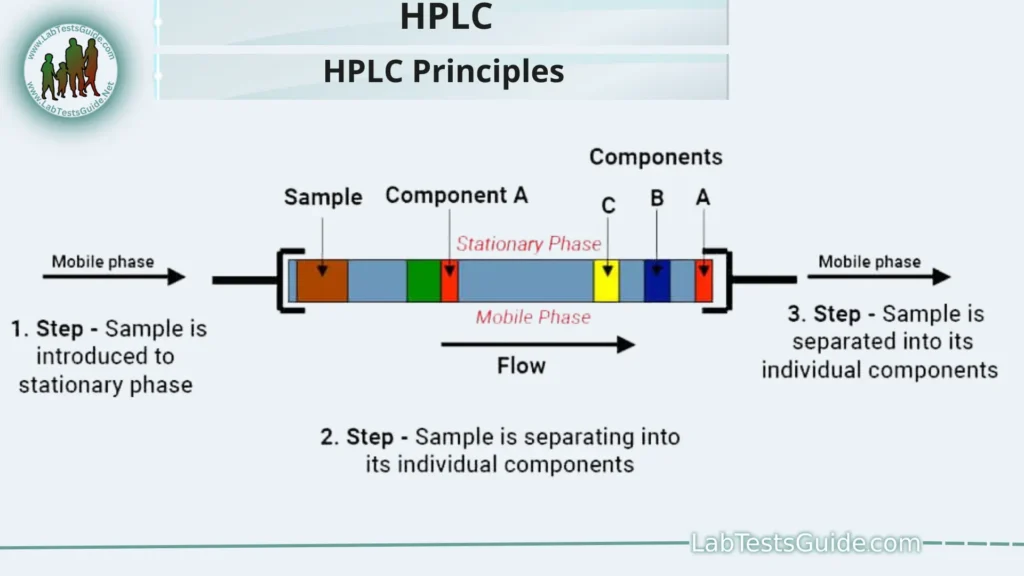
- Chromatographic Separation:At the heart of HPLC lies the principle of chromatographic separation. This separation is achieved by leveraging the differential interactions of sample components with two distinct phases: the stationary phase and the mobile phase. The stationary phase is typically a solid material or a porous gel packed into a column, while the mobile phase is a liquid solvent. Compounds in the sample mixture interact differently with these phases, leading to varying retention times and, consequently, separation.
- Stationary Phase and Mobile Phase:
- Stationary Phase: The stationary phase’s properties, such as surface chemistry and particle size, are carefully chosen to facilitate the separation of specific compounds. Common stationary phases include reversed-phase (hydrophobic), normal-phase (hydrophilic), and ion-exchange materials.
- Mobile Phase: The mobile phase, usually a mixture of solvents, carries the sample through the column. By adjusting the composition and flow rate of the mobile phase, chromatographers can control the separation process. The choice of mobile phase depends on the nature of the analytes and the separation goals.
- Detector Types:Detection is a critical aspect of HPLC. Various detectors are employed to measure analyte concentrations as they elute from the column. Common types of detectors include:
- UV-Visible (UV-Vis) Detector: This detector measures absorbance of UV or visible light by the analytes. It is widely used for compounds that absorb in this range.
- Fluorescence Detector: This detector is sensitive to compounds that fluoresce when exposed to specific wavelengths of light. It offers high sensitivity and selectivity.
- Refractive Index Detector (RID): RID measures changes in the refractive index of the mobile phase caused by analytes. It is particularly useful for compounds with low UV absorbance.
- Mass Spectrometer (MS): When coupled with HPLC (LC-MS), MS provides precise identification and quantification of compounds based on their mass-to-charge ratios. It is highly versatile and used for complex analyses.
Types of HPLC:
High-Performance Liquid Chromatography (HPLC) encompasses various types or modes of operation, each tailored to specific separation needs or analytical objectives. Here are some common types of HPLC:
- Normal-Phase HPLC (NP-HPLC):
- Stationary Phase: Polar (usually silica gel).
- Mobile Phase: Nonpolar solvents.
- Application: Separation based on compound polarity. Suitable for polar compounds with weak to moderate polar interactions.
- Reverse-Phase HPLC (RP-HPLC):
- Stationary Phase: Nonpolar (usually hydrophobic) bonded phase, such as C18.
- Mobile Phase: Polar solvents (e.g., water) mixed with organic solvents (e.g., acetonitrile or methanol).
- Application: Widely used for separating nonpolar and moderately polar compounds. Common in pharmaceutical and chemical analysis.
- Ion-Exchange Chromatography (IEC):
- Stationary Phase: Resins or columns with ion-exchange functional groups (e.g., anion-exchange or cation-exchange).
- Mobile Phase: Aqueous buffer solutions with varying pH.
- Application: Separates ions or charged molecules based on their ionic interactions with the stationary phase. Common in protein purification and analysis of charged species.
- Size-Exclusion Chromatography (SEC) or Gel Filtration Chromatography:
- Stationary Phase: Porous beads with a range of pore sizes.
- Mobile Phase: Aqueous buffer solutions.
- Application: Separates molecules based on their size and shape. Useful for characterizing macromolecules and analyzing polymers.
- Affinity Chromatography:
- Stationary Phase: Immobilized ligands that bind specifically to target molecules (e.g., antibodies).
- Mobile Phase: Buffer solutions.
- Application: Highly selective for purifying and isolating specific biomolecules, such as proteins, enzymes, or antibodies.
- Hydrophilic Interaction Chromatography (HILIC):
- Stationary Phase: Polar but hydrophilic stationary phases.
- Mobile Phase: Mixtures of water and organic solvents.
- Application: Suitable for separating polar and hydrophilic compounds, including highly polar metabolites and glycoproteins.
- Chiral Chromatography:
- Stationary Phase: Chiral stationary phases.
- Mobile Phase: Organic solvents mixed with additives.
- Application: Enantioselective separation of chiral compounds, particularly important in pharmaceutical and agrochemical analysis.
- Supercritical Fluid Chromatography (SFC):
- Stationary Phase: Columns with supercritical carbon dioxide as the primary mobile phase.
- Mobile Phase: Supercritical fluids, often with co-solvents.
- Application: Suitable for separating nonpolar and moderately polar compounds, as well as chiral separations.
- Hyphenated Techniques:
- LC-MS (Liquid Chromatography-Mass Spectrometry): Combines HPLC separation with mass spectrometry detection for precise identification and quantification.
- LC-NMR (Liquid Chromatography-Nuclear Magnetic Resonance): Integrates HPLC with NMR spectroscopy for structural elucidation.
Branches of HPLC:
High-Performance Liquid Chromatography (HPLC) is a liquid chromatography technique, which means it primarily deals with liquid phases (stationary and mobile phases). However, in chromatography, including HPLC, there are two broad categories: liquid chromatography (LC) and gas chromatography (GC). Here’s a brief overview of these two branches:

- Liquid Chromatography (LC):
- HPLC (High-Performance Liquid Chromatography): HPLC uses a liquid mobile phase (commonly a mixture of water and organic solvents) and a liquid stationary phase (commonly packed columns with various stationary phase chemistries). It is ideal for separating a wide range of compounds, including pharmaceuticals, environmental analytes, food components, and more.
- UHPLC (Ultra-High-Performance Liquid Chromatography): UHPLC is an advanced form of HPLC that uses higher pressures and smaller particle size columns to achieve faster separations and improved efficiency.
- LC-MS (Liquid Chromatography-Mass Spectrometry): LC-MS combines liquid chromatography separation with mass spectrometry detection for precise compound identification and quantification. It is commonly used in pharmaceuticals, environmental analysis, and proteomics.
- LC-NMR (Liquid Chromatography-Nuclear Magnetic Resonance): LC-NMR integrates liquid chromatography with NMR spectroscopy for structural elucidation of compounds.
- Gas Chromatography (GC):
- Gas-Liquid Chromatography (GLC): GLC is an older term for gas chromatography. It uses a gaseous mobile phase (carrier gas) and a liquid stationary phase (thin liquid film on a solid support). GC is primarily used for volatile and thermally stable compounds, making it suitable for applications like analyzing volatile organic compounds, environmental analysis, and drug testing.
- GC-MS (Gas Chromatography-Mass Spectrometry): GC-MS combines gas chromatography separation with mass spectrometry detection. It is widely used for the analysis of volatile organic compounds, including drug metabolites, explosives, and environmental contaminants.
HPLC Applications:
High-Performance Liquid Chromatography (HPLC) is a versatile analytical technique with a wide range of applications across various industries. Its ability to separate, identify, and quantify compounds in complex mixtures has made it an indispensable tool in research, quality control, and numerous other fields. Here, we explore some of the prominent applications of HPLC:
- Pharmaceutical Analysis:HPLC is extensively used in the pharmaceutical industry to analyze drug compounds, ensure product quality, and determine drug purity. It plays a vital role in various stages of drug development, from raw material analysis to quality control of finished products. Pharmaceutical scientists rely on HPLC for quantifying active pharmaceutical ingredients (APIs), impurities, and degradation products.
- Environmental Monitoring:Environmental scientists utilize HPLC to detect and quantify pollutants, contaminants, and environmental toxins in water, air, soil, and biological samples. HPLC can identify a wide range of organic and inorganic compounds, making it essential for assessing the environmental impact of industrial processes and ensuring compliance with regulatory standards.
- Food and Beverage Analysis:HPLC is instrumental in the food and beverage industry for analyzing components like vitamins, additives, preservatives, pesticides, and flavor compounds. It aids in ensuring the safety and quality of food products, monitoring for contaminants, and verifying compliance with food safety regulations.
- Clinical Chemistry:In clinical laboratories, HPLC is utilized for analyzing biological samples, including blood, urine, and serum, to determine the concentration of biomarkers, drugs, hormones, and metabolites. It plays a crucial role in disease diagnosis, therapeutic drug monitoring, and medical research.
- Chemical Research:Researchers in academia and industry rely on HPLC for a wide range of chemical analyses. It is used in the isolation and purification of compounds, studying reaction kinetics, characterizing organic and inorganic compounds, and investigating chemical processes.
- Forensic Analysis:Forensic scientists use HPLC to identify and quantify drugs of abuse, explosives, toxic substances, and various compounds found in crime scene evidence. HPLC analysis is crucial for criminal investigations and legal proceedings.
- Biotechnology and Life Sciences:HPLC plays a significant role in biotechnology and life sciences by separating and analyzing biomolecules such as proteins, peptides, nucleic acids, and carbohydrates. This is essential for research in fields like genomics, proteomics, and biopharmaceuticals.
- Quality Control in Manufacturing:Industries such as petrochemicals, cosmetics, and textiles rely on HPLC to monitor the quality of raw materials, intermediate products, and finished goods. It ensures that products meet specified standards and specifications.
- Herbal Medicine and Natural Products:HPLC is used to analyze the chemical composition of herbal medicines, natural products, and dietary supplements. It helps in identifying active compounds, ensuring product consistency, and verifying label claims.
- Petroleum and Petrochemical Analysis:HPLC is employed in the analysis of petroleum products to determine the composition of hydrocarbons, impurities, and additives. This information is crucial for refining processes and meeting regulatory requirements.
HPLC Equipments:
High-Performance Liquid Chromatography (HPLC) relies on a range of specialized equipment and instruments to carry out its intricate separation and analysis processes. Below, we explore the key components and instruments that constitute an HPLC system:

- HPLC Column:The column is one of the central components of the HPLC system. It is packed with a stationary phase that interacts with sample components, causing separation. Columns come in various types, such as reverse-phase, normal-phase, and size-exclusion, each suited to different separation goals.
- Injector:The injector is responsible for introducing the sample into the HPLC system. Samples are typically injected through a syringe or an autosampler, which can handle multiple samples automatically. Precise injection ensures accurate and reproducible results.
- Pump:The pump is responsible for delivering the mobile phase at a controlled flow rate through the system. High-pressure pumps are common in HPLC to maintain the necessary flow rates required for efficient separations.
- Detector:Detectors are critical for measuring and quantifying the compounds eluting from the column. Several types of detectors are used in HPLC, including:
- UV-Visible Detector: Measures absorbance of UV or visible light by analytes.
- Fluorescence Detector: Sensitive to compounds that fluoresce.
- Refractive Index Detector (RID): Measures changes in refractive index.
- Mass Spectrometer (MS): Provides precise identification based on mass-to-charge ratios.
- Column Oven or Heater:Some analyses require temperature control of the column to enhance separation. A column oven or heater maintains a stable temperature, which can improve separation efficiency and consistency.
- Gradient Controller:In gradient elution chromatography, where the composition of the mobile phase changes over time, a gradient controller is used to manage the mobile phase composition. This allows for complex separations and improved peak resolution.
- Data Acquisition and Analysis Software:HPLC systems are controlled by specialized software that not only controls instrument parameters but also collects and analyzes data. These software packages provide tools for peak integration, calibration, and reporting.
- Solvent Reservoirs and Degassers:Solvent reservoirs store the mobile phase solvents, and degassers remove dissolved gases that could interfere with the accuracy of flow rate control.
- Waste Collector:Used mobile phase and waste from the column are collected in a waste container. Proper waste management is essential for laboratory safety and environmental compliance.
- Tubing and Fittings:High-quality tubing and fittings are crucial to ensure a leak-free and consistent flow of the mobile phase and samples throughout the system.
- Pressure Regulator:Pressure regulators maintain a stable system pressure, which is especially important in maintaining the performance of the column.
- Guard Columns:Guard columns are often used to protect the analytical column from particulates and contaminants that can degrade its performance over time.
HPLC Method Development:
High-Performance Liquid Chromatography (HPLC) method development is a crucial process in ensuring the success of an analytical experiment. It involves optimizing various parameters to achieve efficient separation and accurate quantification of target compounds. Here, we explore the key strategies and considerations for HPLC method development:
1. Define the Analytical Objectives:
- Clearly outline the goals of your analysis. What compounds are you trying to separate and quantify? What is the desired level of sensitivity and accuracy? Understanding your objectives is the first step in method development.
2. Selection of Column and Stationary Phase:
- Choose an appropriate HPLC column and stationary phase based on the nature of your analytes. Different columns and phases exhibit varying selectivity, and this choice significantly influences separation.
3. Mobile Phase Selection:
- Select an appropriate mobile phase or solvent system. The choice of solvents, their composition, and pH can impact separation. Consider factors like solubility, polarity, and stability of the analytes.
4. Initial Conditions:
- Set initial HPLC conditions, including column temperature and flow rate. These conditions should be close to the expected optimal values but can be adjusted during method development.
5. Sample Preparation:
- Determine the necessary sample preparation steps, such as dilution, filtration, or derivatization, to ensure accurate and reproducible results. Proper sample preparation can simplify chromatography.
6. Gradient or Isocratic Elution:
- Decide whether to use a gradient elution (changing mobile phase composition over time) or isocratic elution (constant mobile phase composition). Gradients offer more control over separation but may require longer analysis times.
7. Detector Choice:
- Select a suitable detector based on the analytes’ properties. UV-Visible detectors are common for many compounds, but for specialized applications, fluorescence or mass spectrometry may be necessary.
8. Optimization of Parameters:
- Systematically optimize HPLC parameters like flow rate, column temperature, detector wavelength, and gradient profile. This involves experimenting with different conditions to achieve the desired separation and peak shapes.
9. Peak Resolution:
- Ensure adequate peak resolution between target compounds. Adjust column type, mobile phase, or gradient to achieve baseline separation, especially for complex mixtures.
Data Analysis in HPLC:
Data analysis is a critical aspect of HPLC, as it involves interpreting the information gathered during the chromatographic separation and quantifying the compounds of interest. Here are the key components and techniques involved in data analysis for HPLC:
1. Chromatogram Interpretation:
- Peaks: The primary feature in an HPLC chromatogram is the peaks. Each peak represents a compound in the sample, and its properties, such as retention time and peak shape, provide information about the compound’s identity and purity.
- Retention Time: The time it takes for a compound to elute from the column (retention time) is a crucial parameter. It can be used for compound identification and comparison with reference standards.
- Peak Area and Peak Height: The area under the peak (peak area) or the peak’s maximum height (peak height) is directly proportional to the concentration of the compound. Integration software is used to calculate peak areas accurately.
2. Quantitative Analysis:
- Calibration Curve: To quantify the amount of a compound in a sample, a calibration curve is constructed. This curve relates the peak area or height to known concentrations of the compound. By comparing the sample’s peak area to the calibration curve, the concentration can be determined.
- Internal Standards: Internal standards of known concentration are often used to compensate for variations in injection volume and detector response. They aid in accurate quantification.
3. Qualitative Analysis:
- Spectral Data: Some detectors, like UV-Visible detectors, provide spectral information. The shape of the absorption spectrum can help confirm the identity of a compound.
- Co-elution: When two or more compounds elute at the same retention time, it may indicate co-elution. Qualitative analysis can help distinguish and identify these compounds.
4. Data Processing Software:
- Chromatography Data System (CDS): Specialized software is used for data acquisition and processing. These systems enable peak integration, calibration curve generation, and reporting of results.
5. Data Validation:
- System Suitability Tests: Prior to sample analysis, perform system suitability tests to ensure that the HPLC system is operating within specified parameters. These tests assess parameters such as resolution, tailing factor, and plate count.
- Quality Control Standards: Include quality control (QC) standards in each analysis to monitor system performance and verify the accuracy of the results.
6. Reporting and Documentation:
- Results Reporting: Document and report the analysis results, including compound identities, concentrations, and any relevant information, in a standardized format.
- Method Validation: If the analysis is part of a regulated environment (e.g., pharmaceuticals), follow established guidelines for method validation and report the results accordingly.
7. Troubleshooting:
- Peak Shape and Tailing: If peaks exhibit poor shape or tailing, it may indicate issues with the column or mobile phase. Troubleshooting these problems is essential to maintain data quality.
- Baseline Noise: Baseline noise can affect the accuracy of quantification. Identify and mitigate sources of noise in the chromatogram.
8. Data Storage and Archiving:
- Data Integrity: Maintain data integrity by adhering to data storage and archiving protocols, especially in regulated environments where data traceability is critical.
Troubleshooting HPLC:
HPLC is a powerful analytical technique, but like any laboratory method, it can encounter challenges and issues. Effective troubleshooting is essential to identify and resolve these problems promptly. Here are common HPLC issues and strategies for addressing them:
1. Baseline Problems:
- Symptom: A noisy or drifting baseline can obscure peaks.
- Possible Causes and Solutions:
- Contaminated Solvent: Ensure the mobile phase and solvents are free from impurities or particulates. Use high-quality, filtered solvents.
- Air Bubbles: Check for and eliminate air bubbles in the mobile phase lines and degas the solvent properly.
- Leakage: Inspect the system for leaks, especially at connections and fittings. Tighten connections as needed.
- Detector Issues: Clean or maintain the detector as recommended by the manufacturer.
2. Peak Tailing:
- Symptom: Peaks have a tailing or asymmetrical shape.
- Possible Causes and Solutions:
- Column Contamination: Contaminants on the column may cause tailing. Clean or replace the column.
- Incorrect pH: Ensure that the pH of the mobile phase matches the column specifications.
- Injection Volume: Use an appropriate injection volume. Overloading the column can cause tailing.
- Sample Matrix: Sample impurities or matrix effects can cause tailing. Consider sample cleanup or a different sample preparation method.
3. Poor Resolution:
- Symptom: Peaks are not well-separated.
- Possible Causes and Solutions:
- Mobile Phase Composition: Adjust the mobile phase composition, including solvent ratios or gradient programs, to improve separation.
- Column Choice: Consider using a different column phase or size to enhance resolution.
- Column Age: If the column is old or deteriorated, replace it.
4. Peak Splitting:
- Symptom: Single peaks split into multiple smaller peaks.
- Possible Causes and Solutions:
- Mobile Phase Degradation: Mobile phase components may degrade over time. Prepare fresh mobile phase solutions.
- Detector Saturation: If the detector is saturated due to high analyte concentrations, dilute the sample or adjust detector settings.
- Column Overloading: Reduce the sample injection volume or concentration to prevent overloading the column.
5. Loss of Sensitivity:
- Symptom: Reduced or no signal from the detector.
- Possible Causes and Solutions:
- Detector Issues: Check the detector for issues such as lamp replacement or detector cell cleaning.
- Mobile Phase Flow Rate: Ensure that the mobile phase is flowing at the correct rate. A flow rate that is too low can reduce sensitivity.
- Injection Volume: Use an appropriate injection volume to prevent overloading the detector.
6. Retention Time Drift:
- Symptom: Retention times vary from run to run.
- Possible Causes and Solutions:
- Mobile Phase Composition: Check the composition of the mobile phase for consistency.
- Column Temperature: Ensure that the column temperature is stable.
- Column Equilibration: Allow the column to equilibrate at the starting conditions for an appropriate time before each run.
7. Irreproducible Results:
- Symptom: Results are inconsistent between runs.
- Possible Causes and Solutions:
- Sample Preparation: Ensure that sample preparation is consistent and reproducible.
- Column Conditioning: Before sample analysis, condition the column with several injections to stabilize performance.
- Instrument Maintenance: Regularly maintain and calibrate the HPLC system.
Future Developments in HPLC:
The field of High-Performance Liquid Chromatography (HPLC) continues to evolve, driven by advances in technology, increasing analytical demands, and the need for improved efficiency and sensitivity. Here are some potential future developments in HPLC:
- Miniaturization and Microfluidics: Miniaturization of HPLC systems and the incorporation of microfluidic technologies can lead to reduced sample and solvent consumption, faster analysis times, and increased portability, making HPLC more accessible for point-of-care and field applications.
- Enhanced Column Technology: Advances in column packing materials, including smaller particle sizes, novel stationary phases, and improved column chemistries, can lead to higher resolution and more efficient separations.
- Green Chemistry: Development of more environmentally friendly HPLC methods, such as the use of alternative solvents and reduced waste generation, aligning with the principles of green chemistry.
- Automation and Robotics: Greater automation and robotics integration can streamline sample handling, preparation, and analysis, reducing human error and increasing throughput.
- Hyphenated Techniques: Continued growth in hyphenated techniques, such as LC-MS (Liquid Chromatography-Mass Spectrometry) and LC-NMR (Liquid Chromatography-Nuclear Magnetic Resonance), for comprehensive chemical analysis, including structural elucidation and quantification.
- Improved Detectors: Advancements in detector technology, such as more sensitive and selective detectors, can enhance the detection and quantification of analytes, even at lower concentrations.
- Data Handling and Big Data: Enhanced data analysis tools, including machine learning and artificial intelligence algorithms, for automated peak integration, compound identification, and data interpretation, especially in high-throughput applications.
- Portable and Handheld HPLC Devices: Development of portable and handheld HPLC devices for in-field and point-of-care applications, offering rapid and on-site analysis capabilities.
- Multidimensional Chromatography: Wider adoption of multidimensional chromatography techniques to tackle complex sample matrices and improve separation efficiency.
- Advanced Sample Preparation: Innovative sample preparation techniques, such as online sample cleanup and pre-concentration, to reduce matrix effects and improve sensitivity.
- High-Pressure Tandem Systems: Evolution of HPLC into ultra-high-pressure liquid chromatography (UHPLC) and the development of tandem systems that can perform two-dimensional separations to handle complex samples.
- Bioprocess Analytics: Increased focus on HPLC methods for bioprocess analytics, including the analysis of biopharmaceuticals, antibodies, and other biomolecules, to support biopharmaceutical manufacturing.
- Regulatory Compliance: Continued alignment with evolving regulatory requirements, including ensuring data integrity, traceability, and compliance with standards in various industries, such as pharmaceuticals and food safety.
- Customization and Flexibility: More customizable HPLC systems that allow researchers to tailor instrument configurations to their specific needs, accommodating diverse applications.
- Sample Tracking and Traceability: Implementation of advanced sample tracking and traceability solutions to enhance the reliability and integrity of data generated in HPLC laboratories.
HPLC analyzers:
High-Performance Liquid Chromatography (HPLC) analyzers are diverse and specialized instruments designed for various applications. Here is a list of some common types of HPLC analyzers and their applications:
- UV-Visible HPLC Detectors:
- Diode Array Detector (DAD): Measures absorbance across a spectrum of wavelengths, allowing for simultaneous multi-wavelength detection.
- Fixed Wavelength UV-Vis Detector: Measures absorbance at a specific wavelength for routine HPLC analysis.
- Fluorescence HPLC Detector:
- Fluorescence Detector: Detects fluorescent compounds, offering high sensitivity and selectivity, commonly used in pharmaceutical and environmental analysis.
- Refractive Index HPLC Detector:
- Refractive Index Detector (RID): Measures changes in refractive index and is often used for compounds with low UV absorbance, such as sugars and polymers.
- Mass Spectrometry HPLC Analyzers:
- LC-MS (Liquid Chromatography-Mass Spectrometry): Combines HPLC separation with mass spectrometry for precise compound identification and quantification, widely used in pharmaceutical, proteomics, and metabolomics research.
- LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry): Provides enhanced selectivity and sensitivity by using two mass spectrometers in tandem, commonly applied in clinical and environmental analysis.
- Evaporative Light Scattering Detector (ELSD):
- ELSD Detector: Measures the scattering of light by analyte particles, suitable for compounds with low or no UV absorption, such as lipids and certain polymers.
- Conductivity HPLC Detector:
- Conductivity Detector: Measures changes in electrical conductivity caused by ions in the eluent, typically used for ion chromatography applications.
- Electrochemical HPLC Detector:
- Electrochemical Detector: Detects compounds based on their electrochemical properties, often employed in neurotransmitter analysis and pharmaceutical research.
- Pulsed Amperometric Detector (PAD):
- PAD Detector: Specifically designed for the analysis of carbohydrates and sugars, commonly used in the food industry.
- Charge-Coupled Device (CCD) Detector:
- CCD Detector: Utilizes a CCD array to capture images of separated compounds, offering high sensitivity and versatility.
- Viscosity Detector:
- Viscosity Detector: Measures changes in viscosity caused by the presence of analytes, mainly used for analyzing macromolecules like proteins and polymers.
- Flame Ionization Detector (FID):
- FID Detector: Commonly associated with gas chromatography (GC), but can be adapted for HPLC analysis for volatile organic compounds.
- Corona Charged Aerosol Detector (CAD):
- CAD Detector: Measures aerosolized particles produced by compounds eluting from the column, suitable for a wide range of analytes.
FAQs:
What is HPLC?
HPLC stands for High-Performance Liquid Chromatography. It is an analytical technique used for separating, identifying, and quantifying components in a mixture based on their interactions with a stationary phase and a mobile phase.
What are the basic principles of HPLC?
HPLC relies on the principles of chromatographic separation, where a sample is passed through a column packed with a stationary phase. The compounds in the sample interact differently with the stationary and mobile phases, leading to separation based on factors like polarity, size, and chemical properties.
What is the difference between HPLC and GC (Gas Chromatography)?
HPLC uses a liquid mobile phase, while GC uses a gaseous mobile phase. GC is typically used for volatile compounds, while HPLC is suitable for a wider range of analytes, including non-volatile and thermally unstable compounds.
What types of detectors are used in HPLC?
HPLC detectors include UV-Visible detectors, fluorescence detectors, refractive index detectors (RID), and mass spectrometers (LC-MS). The choice of detector depends on the nature of the analytes and the analytical goals.
How is a calibration curve used in HPLC?
A calibration curve relates the peak area or height to known concentrations of a compound. It is used to quantify the concentration of an analyte in a sample by comparing the sample’s peak area to the curve.
What is gradient elution in HPLC?
Gradient elution is a technique where the composition of the mobile phase is changed during the analysis. It is used to optimize separation by adjusting solvent gradients to improve resolution and reduce analysis time.
How do I choose the right HPLC column?
The choice of an HPLC column depends on the properties of the analytes, such as polarity and size, as well as the separation goals. Factors like column material, stationary phase, and column dimensions should be considered.
What is the purpose of sample preparation in HPLC?
Sample preparation is essential to ensure that the sample is compatible with the HPLC method. It may involve steps like filtration, dilution, or extraction to remove impurities and achieve accurate and reproducible results.
What are the common challenges in HPLC analysis?
Common challenges include baseline noise, peak tailing, poor resolution, and issues related to sample preparation. Troubleshooting techniques can be employed to address these challenges.
Is HPLC used in specific industries or applications?
HPLC is widely used in various industries, including pharmaceuticals, environmental analysis, food and beverage, clinical chemistry, and research. It is employed for quality control, research, and regulatory compliance in these sectors.
What are the future developments in HPLC?
Future developments in HPLC may include advances in miniaturization, green chemistry, automation, multidimensional chromatography, and improved detectors to enhance efficiency, sensitivity, and sustainability.
Conclusion:
In conclusion, High-Performance Liquid Chromatography (HPLC) stands as a cornerstone in the realm of analytical chemistry, offering powerful capabilities for separating, identifying, and quantifying compounds across diverse industries. Its enduring principles, coupled with ongoing advancements in technology and methodology, ensure its continued relevance and adaptability in addressing complex analytical challenges. As HPLC continues to evolve, it holds the promise of further enhancing precision, sensitivity, and sustainability, thereby contributing to the advancement of scientific research, quality control, and innovation across multiple disciplines.
Possible References Used





One Comment