Gram staining is initially established by the physician Hans Christian Gram, which was from Denmark. He help to distinguish Klebsiella pneumonia to pneumococci. In short, the process of gram staining comprises the use of a solution of Gram iodine or Potassium iodide to the cells which are use to stain before with Crystal violet or Gentian violet. This technique yields “Iodine stain complex, purple color” in the cytoplasm of micro-organism for example bacteria. The cells that are previously stained with crystal violet and iodine are then treat with a decolorizing reagent like, Ethanol 95% or Acetone alcohol. The variance among Gram positive bacteria and Gram negative bacteria is in their permeability of the cell wall, purple color dye Gram Iodine, when treat with the decolorizing reagent. Though Gram positive keep purple iodine stain later they treat with the decolorizing solution, Gram negative does not keep complexes when it is decolorized. To see gram negative, 1st they are decolorized, a red color counter stain/dye, Safranin is use later decolorization solution treatment.
Smear Preparation:
The 1st thought is the precise training of smear. On a clean and smooth glass slide make a thin film/smear with specimen, with the help of a sterilized wire loop or swab for viscid samples. After air dry of film, heat and fix the smeared slide through passing it many times on a flame (Keep in mind that slide must not be too warm to touch and not burn the back of the hand). If we fail to follow these steps, might that we can create staining arti-facts and interrupt the typical morphology and shape of bacteria and different cells. To be observable on a glass slide, bacteria which are stained by the Gram staining procedure, it is essentially that concentrations of at least of 104 – 10 bacteria/micro liter of concentrated staining reagent.

At lesser concentrations, the Gram stain solution of a clinical samples infrequently tells bacteria/organisms even, if the culture is positive in microbiology laboratory. Film which is not accurately made and fixed have a tendency to to be rinse away during the staining process and washing follow on the lack of stained organisms. In exceptional cases, the following rules are helpful: If a cerebrospinal fluid received and consists of only a little/few bacteria, they are more possible to be found if a concentrated and a Thick Smear is observed. To make/prepare a Thick Smear, the sample is rotated at maximum speed and a big drop of specimen sediment is used and pour in the middle of the glass slide and air dry. The Cyto-centrifuge can demonstrate to be helpful in concentrating organisms as well as in conserving cells shape and morphology. When fluid samples for example urine or CSF is receive it look to disappear after the staining process, a wax spot, positioned near the smeared part on the Same side on the glass slide after the staining process (to escape from wax artifacts) will decrease prevention in finding the sample below the microscope. The wax spot maybe use for rapid focusing. In a grossly/clearly blood sample, it demonstrate hard to differentiate micro-organisms or bacteria from artifacts. After air dry the smear and heat fix of these type of samples, the additional introductory phase of cover it with distilled water, wait for 5 minutes, and then washing, can cause the RBCs to break/lyse and float off.
Preparation of Gram Stain Reagents
Basic Stain ===> Crystal Violet
Solution A
- Crystal violet 2 grams
- Ethyl alcohol 20 micro liters
Solution B
- Ammonium oxalate 8 gram
- Distilled water 80 micro liters
- After mixing the Solution A and Solution B. Save them for 24 hours or 1 day and filter. Stock in an Amber color
Mordant ====> Gram’s Iodine
- Iodine 1 gram
- Potassium iodide 2 grams
- Distilled water 100 micro liters
- Mixing and Stock in an Amber color
Decolorizer ====> Ethanol 95% 1:1 with Ethanol and Acetone
- Acetone 50 micro liters
- Ethanol (95%) 50 micro liters
Counterstain ====> Safranin
- Safranin O 34 gram
- Absolute alcohol 10 micro liters
- Distilled water 90 micro liters
- Mixing, filter and stock in Amber color
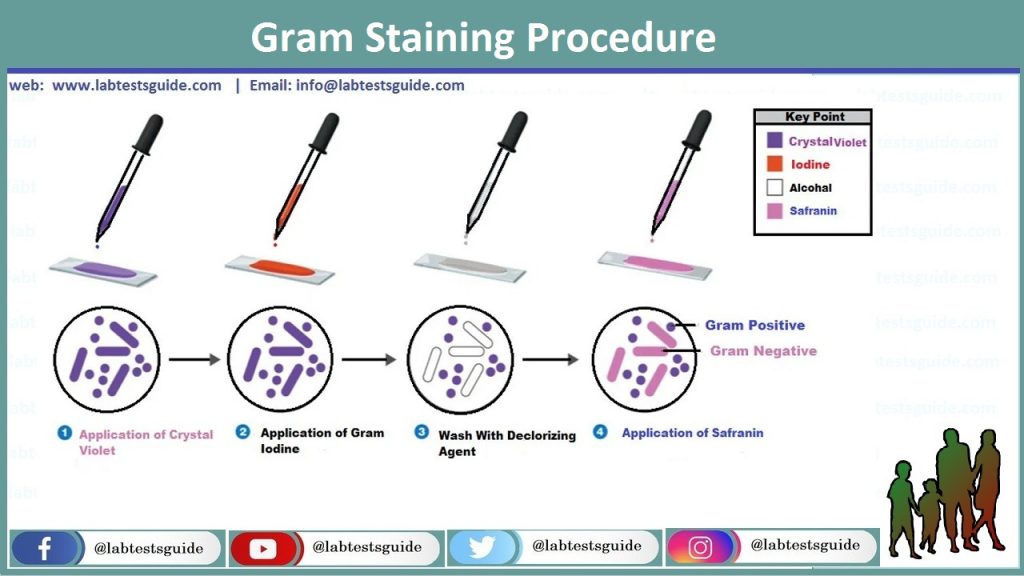
Gram Staining Procedure:
- Pour the slide with Crystal violet or Gentian solution for 1 Min or 60 Seconds.
- Rinse the slide in running tap water drop by drop on the back of the hand on slide so that the smear will not wash away.
- Pour the slide with Gram’s iodine for at least 2 Min or 180 Seconds.
- Again rinse the slide in running tap water.
- Sensibly cover with Decolorizer solution, 95% Ethanol.

- Wash with running tap water. This 3rd step is the most precarious and crucial also the one maximum affected through practical changes in timing and reagents. Decolorizer is use only for 15 Seconds.
- Pour the slide with Counter stain safranin or 10% Fuchsine for 1 Min or 60 Seconds.
- Rinse in tap water.
- Air dry the glass slide, or blot by Absorbent paper.
Results:
Bacteria retain the crystal violet and gram iodine mixtures complexes after the wash in 95% ethanol solution in purple color and are named as Gram positive Bacteria, those which miss these complexes of solution are red in color by counter stain the safranin or 10% fuchsine are named as Gram negative Bacteria.

Examples of Gram Positive Bacteria:
Staphylococcus spp, Streptococcus spp, Bacillus spp, Nocardia, Clostridium spp etc.
Examples of Gram Negative Bacteria:
Escherichia spp, Salmonella spp, Shigella spp, Pseudomonas, Neisseria spp, Klebsiella, Enterobacter spp etc.

Relationship between Gram Staining and Cell Wall Structure of Bacteria:
Some the structural changes between the cell walls of gram positive bacteria and gram negative answerable in a way for the Gram stain response? In the Gram staining, an impenetrable crystal violet with gram iodine complexes is made inside the cell of bacteria, and this complexes is extracted by alcohol from gram negative bacteria but not from gram positive. Gram positive organisms are dehydrated by alcohol, they have very dense cell wall containing of many coatings of peptido-glycan. This creates the pores/holes in the cell wall to near, avoiding the insoluble crystal violet + gram iodine complexes from evasion. In gram negative alcohol eagerly infiltrates the lipid rich external layer, and the thin peptido-glycan coating does not stop solvent channel, therefore, the crystal violet + gram iodine complexes is simply detached or removed.
| Gram stain procedural steps. | ||
|---|---|---|
| Step | Procedure | Outcome |
| Primary stain(crystal violet) | Add several drops of crystal violet to the smear and allow it to sit for 1 minute. Rinse the slide with water. | Both Gram-positive and Gram-negative cells will be stained purple by the crystal violet dye. |
| Mordant (iodine) | Add several drops of iodine to the smear and allow it to sit for 1 minute. Rinse the slide with water. | Iodine “sets” the crystal violet, so both types of bacteria will remain purple. |
| Decolorization (ethanol) | Add drops of ethanol oneatatime until the runoff is clear. Rinse the slide with water. | Gram-positive cells resist decolorization and remain purple. The dye is released from Gram-negative cells. |
| Counterstain(safranin) | Add several drops of safranin to the smear and allow it to sit for one minute. Rinse the slide with water and blot dry. | Gram-negative cells will be stained pink by the safranin. This dye has no effect on Gram-positive cells, which remain purple. |
Related Articles:
Possible References Used