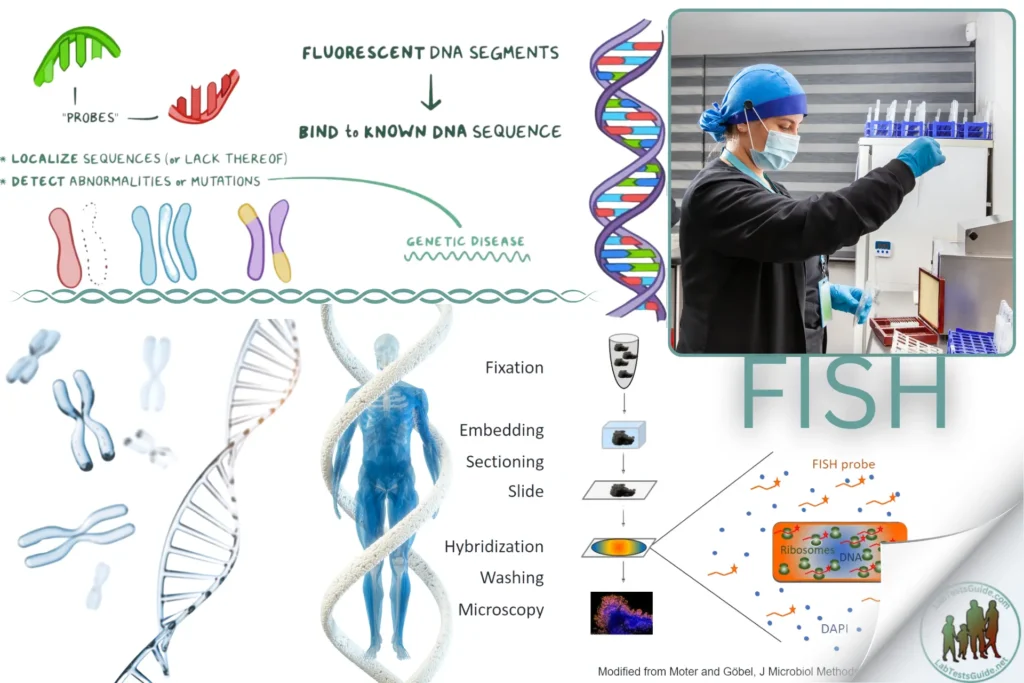
Fluorescence In Situ Hybridization (FISH) is a molecular cytogenetic technique used to detect and localize the presence or absence of specific DNA sequences on chromosomes. It is widely used in clinical diagnostics for identifying chromosomal abnormalities, gene mapping, and detecting gene rearrangements and translocations in cancer.
Introduction
Fluorescence In Situ Hybridization (FISH) is a molecular cytogenetic technique used to detect and localize the presence or absence of specific DNA sequences on chromosomes. It is widely used in clinical diagnostics for identifying chromosomal abnormalities, gene mapping, and detecting gene rearrangements and translocations in cancer.
Principle
FISH utilizes fluorescently labeled DNA probes that hybridize to complementary DNA sequences on chromosomes. After hybridization, the probes can be visualized under a fluorescence microscope, allowing for the identification and localization of specific genetic material.
Specimen Requirements
- Type of specimen: Blood, bone marrow, tissue biopsies, cytology samples (e.g., from PAP smears), or paraffin-embedded tissue sections
- Volume of specimen required: Depends on the type of specimen (e.g., 1-3 mL of blood, several tissue sections)
- Collection method: Standard clinical procedures for sample collection (e.g., venipuncture, biopsy)
- Handling and storage requirements: Fresh samples should be processed promptly or stored at 4°C for short-term; fixed specimens should be stored at room temperature
- Stability of the specimen: Fresh specimens should be processed within 24-48 hours; fixed specimens are stable for several weeks to months
Reagents and Materials:
- Fluorescently labeled DNA probes specific to the target sequences
- Hybridization buffer
- Denaturation buffer
- Wash buffers (e.g., SSC buffer)
- Counterstain (e.g., DAPI for nuclear staining)
- Mounting medium (anti-fade)
- Glass slides and coverslips
- Fixatives (e.g., formaldehyde, methanol/acetic acid)
Equipment:
- Fluorescence microscope with appropriate filters
- Hybridization oven or incubator
- Micropipettes and tips
- Thermocycler (for probe denaturation)
- Humidity chamber
- Centrifuge (for cell samples)
- Slide warmer or hot plate
Procedure:
Sample Preparation:
- For Cell Samples:
- Harvest cells from blood, bone marrow, or cell culture.
- Centrifuge the cells and resuspend in a hypotonic solution to swell them.
- Fix the cells in methanol/acetic acid (3:1) and drop them onto glass slides.
- For Tissue Samples:
- Section paraffin-embedded tissue blocks to 4-5 µm thickness.Place the sections on glass slides and deparaffinize using xylene and ethanol washes.Rehydrate the sections in decreasing concentrations of ethanol.
Denaturation and Hybridization:
- Denature the DNA in the sample by placing the slides in denaturation buffer at 70-80°C for 2-5 minutes.
- Apply the fluorescently labeled DNA probe to the denatured sample on the slide.
- Cover the slide with a coverslip and seal the edges with rubber cement or similar adhesive.
- Place the slides in a humidified chamber and incubate at 37°C overnight to allow hybridization.
Post-Hybridization Washes:
- Remove the coverslip and wash the slides in a series of SSC buffers at room temperature or 42°C to remove non-specifically bound probes.
- Rinse the slides briefly in distilled water.
Counterstaining and Mounting:
- Counterstain the chromosomes with DAPI or another nuclear stain.
- Apply a drop of anti-fade mounting medium to the slide and cover with a coverslip.
- Seal the edges of the coverslip with nail polish to prevent drying.
Quality Control:
- Include positive and negative controls to validate the hybridization efficiency and specificity of the probes.
- Ensure proper calibration and maintenance of the fluorescence microscope.
- Check for non-specific binding and background fluorescence in control samples.
Calculation and Interpretation
- Examine the slides under a fluorescence microscope equipped with the appropriate filter sets.
- Analyze the fluorescence signals to identify the presence, absence, or location of the target DNA sequences.
- Compare the observed fluorescence patterns with normal controls to detect abnormalities such as deletions, duplications, translocations, or gene amplifications.
Limitations
- FISH requires high-quality specimen preparation and precise hybridization conditions.
- Interpretation can be challenging in cases of weak or ambiguous signals.
- The technique is limited by the resolution of the fluorescent probes and the microscope.
Safety Precautions
- Wear appropriate PPE (gloves, lab coat, safety goggles).
- Handle all reagents and samples according to standard biohazard protocols.
- Dispose of waste according to local regulations.
- Work in a well-ventilated area when using organic solvents.
References
- Tietz Textbook of Clinical Chemistry and Molecular Diagnostics.
- Fluorescence In Situ Hybridization (FISH) Protocols (Humana Press).
- Manufacturer’s instructions for FISH probes and reagents.
- Clinical Laboratory Standards Institute (CLSI) guidelines.
This format can be adapted for various types of FISH applications by adjusting the specific details to match the methodology used for each type of assay.
Possible References Used