Enzyme Immunoassay (EIA) is a widely used laboratory technique that combines the principles of immunology and enzymology to detect the presence or quantify the concentration of specific molecules, such as antigens or antibodies, in a sample. EIA is also commonly known as Enzyme-Linked Immunosorbent Assay (ELISA), which is a specific type of EIA.

The basic concept of EIA involves the use of antibodies, which are proteins produced by the immune system in response to foreign substances (antigens), to specifically bind to the target molecule of interest. By utilizing enzymes that can produce detectable signals, such as color changes or fluorescence, the presence or concentration of the target molecule can be determined.
Definition and Purpose:
Definition of Enzyme Immunoassay (EIA):
Enzyme Immunoassay (EIA) is a laboratory technique that combines immunological principles with enzymatic reactions to detect and quantify the presence of specific molecules, such as antigens or antibodies, in a sample. EIA involves the use of antibodies, which are proteins produced by the immune system in response to foreign substances, to selectively bind to the target molecule of interest. The interaction between the antibodies and the target molecule is then detected through an enzymatic reaction, leading to a measurable signal that indicates the presence or concentration of the target molecule.
Purpose of Enzyme Immunoassay:
The primary purpose of EIA is to accurately detect and quantify the presence of specific molecules in a sample. This technique has a wide range of applications across various fields due to its high sensitivity, specificity, and versatility. Some key purposes and applications of EIA include:
- Clinical Diagnostics: EIA is extensively used in medical laboratories to diagnose diseases and monitor health conditions. It can detect various biomarkers associated with diseases such as infections, autoimmune disorders, cancer, hormonal imbalances, and more.
- Research and Biotechnology: EIA plays a crucial role in research by enabling scientists to study molecular interactions, detect protein expression, and analyze biological samples. It is often used to validate the presence of specific molecules in experimental settings.
- Pharmaceutical Development: EIA is utilized in drug development and testing to quantify the concentration of drug compounds, antibodies, or other relevant molecules in biological samples, helping researchers evaluate the effectiveness of potential drugs.
- Food Safety and Environmental Monitoring: EIA is employed to detect contaminants, pathogens, allergens, and toxins in food products and environmental samples. This aids in ensuring the safety and quality of food and assessing environmental conditions.
- Blood Banking and Transfusion Medicine: EIA is used in blood typing and crossmatching procedures to identify blood group antigens and antibodies. This information is crucial for safe blood transfusions.
- Pregnancy Testing: Home pregnancy tests utilize EIA to detect the presence of a hormone called human chorionic gonadotropin (hCG) in urine samples, indicating pregnancy.
- Allergy Testing: EIA is employed in allergen-specific IgE testing to identify allergens that trigger allergic reactions in individuals.
- Hormone Assays: EIA is used to measure hormone levels in clinical samples, aiding in the diagnosis and monitoring of endocrine disorders.
- Virology and Immunology: EIA is pivotal in detecting viral infections, studying immune responses, and assessing vaccination effectiveness.
- Quality Control in Industry: EIA is applied in industries such as pharmaceuticals, biotechnology, and manufacturing to ensure product quality and consistency.
Basic Principles of EIA:
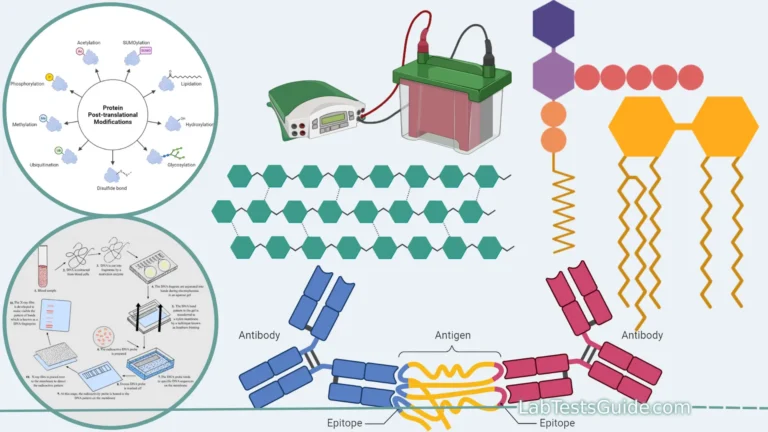
- Immunological Recognition: EIA relies on the highly specific interactions between antibodies and antigens. Antibodies are proteins produced by the immune system in response to the presence of foreign substances, known as antigens. Each antibody is designed to recognize and bind to a particular antigen with a high degree of specificity.
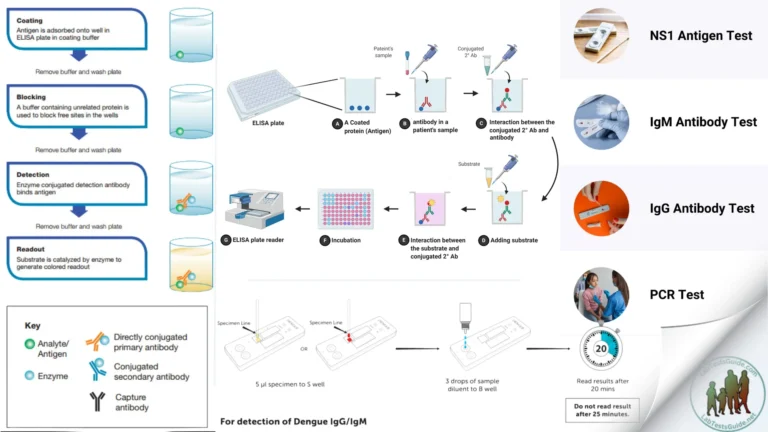
- Antigen-Antibody Binding: In EIA, a solid surface (such as a microtiter plate well) is coated with either the antigen or the antibody of interest. When a sample is added to the coated surface, if the target molecule (antigen) is present in the sample, it will bind to the immobilized antibody (or vice versa, in the case of detecting antibodies). This interaction forms the basis of the assay.
- Enzyme Labeling: To detect the antigen-antibody interaction, an enzyme is conjugated (linked) to either the primary antibody (direct method) or a secondary antibody (indirect method). The enzyme serves as a label that amplifies the signal generated by the binding event.
- Enzymatic Reaction and Signal Generation: After the antigen-antibody interaction occurs, the plate is washed to remove any unbound molecules. A substrate solution containing a substrate specific to the conjugated enzyme is added. The enzyme catalyzes the conversion of the substrate into a detectable product. The nature of the product depends on the enzyme used. For example, in a colorimetric EIA, the enzyme may catalyze a reaction that produces a colored product.
- Signal Measurement: The intensity of the signal generated (e.g., color change) is proportional to the amount of the target molecule present in the sample. The signal can be measured using appropriate instruments like a microplate reader, spectrophotometer, or fluorometer.
- Calibration Curve and Quantification: To quantify the concentration of the target molecule in the sample, a series of standards with known concentrations are run alongside the samples. The signal generated by these standards is used to create a calibration curve, which establishes a relationship between signal intensity and concentration. The signal from the unknown samples can then be compared to the curve to determine their concentrations.
Types of EIA:
- Direct ELISA (Enzyme-Linked Immunosorbent Assay): In a Direct ELISA, the antigen of interest is directly immobilized on a solid surface (usually a microtiter plate well). A labeled enzyme-conjugated antibody specific to the antigen is then added, which binds to the immobilized antigen. The signal generated by the enzyme-conjugated antibody is directly proportional to the amount of antigen present in the sample.Advantages: Simplicity, speed, and reduced risk of cross-reactivity. Limitations: Limited sensitivity compared to other methods.
- Indirect ELISA: In Indirect ELISA, the primary antibody (usually from the host species where the secondary antibody comes from) is used to bind the antigen of interest. Then, a labeled secondary antibody that is specific to the primary antibody is added. This secondary antibody amplifies the signal by binding to the primary antibody.Advantages: Amplified signal, flexibility in using different primary antibodies, and high sensitivity. Limitations: More complex procedure, possibility of non-specific binding.
- Sandwich ELISA: Sandwich ELISA is used for detecting antigens that have two antigenic epitopes. The solid surface is coated with a capture antibody specific to one epitope. The sample containing the antigen is added, and the antigen binds to the capture antibody. Then, a labeled detection antibody specific to a different epitope on the antigen is added, forming a “sandwich” of antigen between the two antibodies.Advantages: High specificity, reduced interference from sample components, high sensitivity. Limitations: Requires two specific antibodies, potential steric hindrance.
- Competitive ELISA: Competitive ELISA is used for detecting small molecules or haptens that are not immunogenic enough to generate antibodies. A known quantity of labeled antigen (with enzyme conjugation) is mixed with the sample containing the unlabeled antigen. They compete for binding to a limited amount of immobilized antibodies. The signal is inversely proportional to the concentration of the unlabeled antigen in the sample.Advantages: Suitable for small molecules, adaptable to various analytes. Limitations: Requires known labeled antigen, sensitivity can be lower for small molecules.
- Reverse ELISA: In Reverse ELISA, the labeled antigen is immobilized on the solid surface, and the specific antibody in the sample binds to it. This type is less common and is primarily used in specialized situations.Advantages: Unique approach, can be useful for certain applications. Limitations: Less widely used, specificity challenges.
Components of EIA:
An Enzyme Immunoassay (EIA) involves several components that contribute to the successful execution of the assay and accurate detection of the target molecule. Here are the key components of an EIA:
- Solid Surface: A solid surface is required to immobilize the components of the assay. This is usually a microtiter plate well, which can be made of plastic or glass and contains multiple wells for running multiple samples simultaneously.
- Coating and Blocking Agents: Coating involves attaching either the antigen or the capture antibody to the solid surface. A blocking agent, often bovine serum albumin (BSA) or milk powder, is used to prevent non-specific binding of other molecules to the coated surface, reducing background noise.
- Antibodies: Antibodies are crucial components in EIA. These include:
- Capture Antibody: Immobilized on the solid surface, it binds to the target antigen.
- Primary Antibody: Binds to the target antigen in the sample.
- Secondary Antibody: Conjugated to an enzyme, it binds to the primary antibody and amplifies the signal.
- Antigen or Sample: The sample being tested contains the target molecule (antigen or antibody) that the assay aims to detect and quantify.
- Enzyme-Conjugated Secondary Antibody: This secondary antibody is conjugated to an enzyme, such as horseradish peroxidase (HRP) or alkaline phosphatase (AP). It binds to the primary antibody-antigen complex and catalyzes the enzymatic reaction.
- Substrate Solution: The substrate solution contains a substrate specific to the enzyme used. When the enzyme-conjugated secondary antibody interacts with the substrate, it catalyzes a reaction that produces a detectable product, such as a colored or fluorescent compound.
- Washing Buffer: Washing steps are essential to remove unbound molecules and reduce background noise. A washing buffer is used to carefully wash the wells between various steps of the assay.
- Calibration Standards: A set of known concentrations of the target molecule (antigen or antibody) are included in the assay. These standards create a calibration curve that helps relate the signal generated to the concentration of the target molecule in unknown samples.
- Microplate Reader or Detection Instrument: A microplate reader, spectrophotometer, or fluorometer is used to measure the intensity of the signal produced by the enzymatic reaction. The instrument quantifies the absorbance, fluorescence, or luminescence, which corresponds to the concentration of the target molecule in the sample.
- Data Analysis Software: Software is used to analyze the data collected from the microplate reader. It helps generate calibration curves, calculate concentrations, and interpret results.
- Pipettes and Consumables: Precision pipettes and disposable tips are used to handle and transfer small volumes of reagents and samples.
Procedure of EIA:
The procedure of an Enzyme Immunoassay (EIA) involves several sequential steps, each designed to facilitate the specific binding of antibodies and antigens and the subsequent detection of the enzymatic signal. Here’s a general outline of the EIA procedure:
- Coating the Plate:
- Coat the microtiter plate wells with the capture antibody or antigen. This immobilizes one component of the antigen-antibody interaction.
- Blocking Non-Specific Binding:
- Add a blocking agent (such as BSA or milk powder) to the plate to prevent non-specific binding of other molecules to the coated surface.
- Sample and Standards Addition:
- Add the samples containing the target antigen (or antibody) and a set of known standards with varying concentrations of the target molecule to separate wells.
- Primary Antibody Incubation:
- Add the primary antibody specific to the target molecule. Allow the plate to incubate, allowing the primary antibody to bind to the target antigen in the sample.
- Washing Steps:
- Wash the plate wells multiple times with a washing buffer to remove unbound components and reduce background noise.
- Secondary Antibody and Conjugation:
- Add the enzyme-conjugated secondary antibody. This antibody binds to the primary antibody-antigen complex, creating a “sandwich” and introducing the enzyme label.
- Substrate Addition and Signal Generation:
- Add the substrate solution specific to the enzyme label. The enzyme catalyzes a reaction that converts the substrate into a detectable product (e.g., a colored or fluorescent compound).
- Signal Detection:
- Use a microplate reader or appropriate detection instrument to measure the intensity of the signal (absorbance, fluorescence, or luminescence) generated in each well.
- Calibration Curve and Data Analysis:
- Use the known concentrations of standards to create a calibration curve relating signal intensity to target molecule concentration.
- Quantification of Samples:
- Compare the signal generated by the unknown samples to the calibration curve to determine their concentrations.
- Interpretation of Results:
- Analyze the calculated concentrations of the target molecule in the samples. Results can be qualitative (presence/absence) or quantitative (actual concentration).
- Data Recording and Reporting:
- Record the results and generate a report, which may include sample identifiers, concentrations, and any relevant notes.
Applications of EIA:
- Clinical Diagnostics:
- Disease Detection: EIA is used to diagnose various medical conditions, including infectious diseases (HIV, hepatitis), autoimmune disorders (rheumatoid arthritis, lupus), and cancer (tumor markers).
- Hormone Assays: EIA quantifies hormones like insulin, thyroid hormones, and reproductive hormones for diagnosing endocrine disorders.
- Pharmaceutical Industry:
- Drug Development: EIA assesses drug levels, pharmacokinetics, and pharmacodynamics during drug development.
- Immunogenicity Testing: EIA detects immune responses to therapeutic drugs, particularly in the context of biologics and vaccines.
- Biotechnology and Research:
- Protein Expression Analysis: EIA helps quantify protein expression levels and assess the impact of genetic modifications.
- Molecular Interaction Studies: EIA measures interactions between proteins, peptides, nucleic acids, and other molecules, aiding in understanding cellular processes.
- Food Safety and Environmental Monitoring:
- Allergen Detection: EIA identifies allergenic proteins in food products, ensuring accurate labeling and preventing allergic reactions.
- Pathogen Detection: EIA detects pathogens (bacteria, viruses) in food and water, contributing to food safety and environmental monitoring.
- Blood Banking and Transfusion Medicine:
- Blood Typing: EIA determines blood group antigens, aiding in safe and compatible blood transfusions.
- Screening for Disease Agents: EIA identifies agents like HIV, hepatitis, and syphilis in donated blood to prevent transmission.
- Virology and Immunology:
- Viral Load Measurement: EIA quantifies viral loads, aiding in monitoring viral infections and assessing the effectiveness of antiviral treatments.
- Immune Response Evaluation: EIA measures antibody levels to evaluate immune responses, vaccine effectiveness, and immunity status.
- Pregnancy Testing:
- Home Pregnancy Tests: EIA detects human chorionic gonadotropin (hCG) in urine, confirming pregnancy.
- Environmental Monitoring:
- Toxin Detection: EIA identifies pollutants, toxins, and contaminants in water, soil, and air samples.
- Quality Control in Industry:
- Pharmaceutical Manufacturing: EIA ensures consistent product quality, assessing protein content and purity.
- Food Production: EIA verifies the presence of specific ingredients and contaminants in food products.
- Autoimmune Disease Testing:
- EIA helps diagnose autoimmune diseases like celiac disease, multiple sclerosis, and systemic lupus erythematosus.
- Drug Abuse Testing:
- EIA screens for the presence of drugs of abuse (such as opioids, cannabinoids, and amphetamines) in biological samples.
Advantages and DisAdvantages:
Enzyme Immunoassay (EIA) offers several advantages and disadvantages, which influence its selection for various applications. Here’s an overview of the pros and cons of EIA:
Advantages of EIA:
- High Sensitivity and Specificity: EIA can detect even low concentrations of target molecules with high specificity due to the specific antigen-antibody interactions.
- Quantitative Analysis: EIA allows for quantitative measurement of target molecules, providing concentration information.
- Wide Range of Applications: EIA is versatile and applicable across diverse fields such as clinical diagnostics, research, food safety, and environmental monitoring.
- Multiplexing Capability: EIA can be adapted for multiplex assays, enabling simultaneous detection of multiple analytes in a single sample.
- Automation and High Throughput: EIA can be automated, allowing for the analysis of many samples simultaneously, increasing efficiency.
- Non-Invasive Testing: In certain applications like pregnancy tests, urine samples can be used, providing a non-invasive means of diagnosis.
- Established Protocols: Standardized protocols and commercially available kits make EIA accessible and reproducible.
Disadvantages of EIA:
- False Positives and Negatives: Non-specific binding or cross-reactivity can lead to false results if not carefully controlled.
- Complex Sample Handling: Some samples may require extensive preparation and purification to minimize interference.
- Limited Dynamic Range: EIA’s sensitivity can sometimes lead to saturation at high concentrations or an inability to detect extremely low levels.
- Need for Specific Antibodies: The availability of specific antibodies is essential for target recognition, limiting its use for molecules with no suitable antibodies.
- Labor-Intensive: While automation is possible, the manual steps involved in EIA can make it labor-intensive, especially when analyzing large numbers of samples.
- Skill-Dependent: Proper execution of EIA requires careful technique and adherence to protocols, which can be challenging in inexperienced hands.
- Sample Matrix Effects: Complex samples with various components might interfere with antigen-antibody interactions or enzymatic reactions.
- Cost: While widely used, the cost of reagents, equipment, and skilled personnel can contribute to the expense of EIA.
Limitations of EIA:
Enzyme Immunoassay (EIA) has several limitations that need to be considered when designing and interpreting experiments. Here are some of the main limitations of EIA:
- Cross-Reactivity: EIA can sometimes show cross-reactivity with molecules similar in structure to the target antigen. This can lead to false-positive results when other substances in the sample share epitopes with the target antigen.
- False Negatives: Non-specific binding or other factors might lead to false-negative results, where the actual presence of the target antigen is not detected.
- Sensitivity Saturation: EIA’s sensitivity can reach a limit where very high concentrations of the target molecule can cause saturation of the enzymatic reaction, resulting in a plateau in the signal.
- Dynamic Range: EIA might not cover a broad dynamic range, making it less suitable for detecting both very low and very high concentrations of the target in a single assay.
- Sample Matrix Interference: Complex samples like serum, plasma, or cell lysates can contain components that interfere with antigen-antibody interactions or enzymatic reactions, affecting accuracy.
- Antibody Availability: The success of EIA relies on the availability of specific and high-affinity antibodies. If these antibodies are not available, the assay cannot be performed.
- Non-Quantitative: While EIA is often used for quantitative analysis, certain variations might be semi-quantitative or qualitative, limiting their precision.
- Labor-Intensive: EIA can involve multiple manual steps, leading to variability and making it more labor-intensive compared to other automated assays.
- Limited Applicability to Small Molecules: EIA is better suited for larger molecules like proteins and antibodies. Detection of small molecules (haptens) might require specialized techniques.
- Time-Consuming: Each step of EIA, including coating, incubation, washing, and signal development, requires a specific duration, which can make the assay time-consuming.
- User Variability: Operator technique can influence the consistency and reliability of results, making proper training and adherence to protocols crucial.
- Cost: EIA can be expensive due to the need for specialized reagents, equipment, and the time and expertise required to perform the assay.
- Limited Multiplexing: While multiplexing is possible with EIA, the number of targets that can be simultaneously detected in a single well is limited compared to newer technologies.
ELISA vs EIA:
| spect | ELISA (Enzyme-Linked Immunosorbent Assay) | EIA (Enzyme Immunoassay) |
|---|---|---|
| Definition | A specific type of EIA that uses solid-phase immunoassays with enzymes as labels for detection. | A broader term encompassing assays that use enzymes for detection of specific antigens or antibodies. |
| Types | Direct ELISA, Indirect ELISA, Sandwich ELISA, Competitive ELISA, etc. | Direct EIA, Indirect EIA, Sandwich EIA, Competitive EIA, etc. |
| Enzyme Labeling | Enzymes are used as labels, typically conjugated to secondary antibodies or antigens. | Enzymes are used as labels to generate a signal for target detection. |
| Solid Surface | Solid surfaces (microtiter plate wells) are coated with antigens or antibodies. | Solid surfaces (microtiter plate wells) are often used, but the focus is on enzyme-based detection. |
| Signal Generation | Enzymes catalyze reactions to produce a detectable product (color, fluorescence, luminescence). | Enzymes catalyze reactions to produce a signal proportional to the target concentration. |
| Common Usage | Commonly used term when discussing plate-based assays for antigen or antibody detection. | A more general term used to describe assays using enzymes for detecting specific molecules. |
| Example Scenario | Detecting a specific protein in a patient’s serum using a sandwich ELISA. | Detecting the presence of an allergen in a food sample using a competitive EIA. |
| Specific Context | Often used interchangeably with “EIA” in the context of enzyme-based immunoassays. | Used to refer to a broader range of immunoassays that incorporate enzyme labels. |
Comparison to Other Assay Methods:
| Aspect | Enzyme Immunoassay (EIA) | Polymerase Chain Reaction (PCR) | Western Blotting |
|---|---|---|---|
| Purpose | Detect and quantify antigens or antibodies using enzyme labels. | Amplify and detect specific DNA sequences. | Detect and analyze specific proteins. |
| Detection Target | Antigens, antibodies, proteins. | DNA sequences. | Proteins. |
| Detection Method | Enzymatic reaction (color, fluorescence, luminescence). | Amplification of DNA sequences using thermal cycling. | Antibodies binding to target proteins. |
| Labeling | Enzymes (e.g., HRP, AP) | Fluorescent dyes, DNA probes. | Antibodies, often labeled. |
| Detection Sensitivity | High sensitivity, can detect low concentrations. | High sensitivity, can detect a single DNA molecule. | Moderate sensitivity. |
| Quantitative or Qualitative | Both quantitative and qualitative detection. | Mostly qualitative, can be semi-quantitative. | Mostly qualitative, semi-quantitative with analysis. |
| Sample Type | Various sample types (serum, plasma, cell lysates, etc.). | DNA or RNA samples. | Protein lysates or tissue extracts. |
| Automation Possibility | Automation feasible for high-throughput assays. | Automation for amplification, not as much for detection. | Semi-automated detection. |
| Applications | Clinical diagnostics, research, food safety, etc. | Genetic analysis, diagnostics, genomics research. | Protein expression analysis, research. |
| Time Required | Several hours to a day, depending on the assay type. | A few hours to a day for amplification and detection. | A few hours to a day for protein separation and detection. |
| Equipment Required | Microplate reader, detection instrument. | Thermal cycler, gel electrophoresis equipment. | Gel electrophoresis, transfer equipment. |
| Cost | Moderately expensive due to reagents, instruments. | Moderately expensive due to reagents, equipment. | Equipment and reagents costs. |
| Analysis Complexity | Moderate to high due to multiple steps and data interpretation. | Moderate, requires some technical expertise. | Moderate, requires proper protocol adherence. |
| Multiplexing Capability | Limited multiplexing capabilities. | Limited to multiplex PCR techniques. | Limited to the number of antibodies used. |
| Sample Throughput | High throughput possible with automation. | Moderate throughput due to cycling time. | Moderate throughput for analysis. |
Future Developments in EIA:
The field of Enzyme Immunoassay (EIA) continues to evolve with advancements in technology and scientific understanding. Here are some potential future developments in EIA:
- Nanotechnology Integration: Nanoparticles and nanomaterials can enhance EIA sensitivity and enable multiplexing. They offer increased surface area for immobilization, improved signal amplification, and reduced assay times.
- High-Dimensional Assays: Multi-omics approaches, such as combining EIA with genomics, proteomics, and metabolomics, could provide comprehensive insights into complex biological systems and disease mechanisms.
- Miniaturization and Point-of-Care Devices: Miniaturized EIA platforms, including microfluidic devices and lab-on-a-chip systems, could enable rapid, on-site diagnostics in remote or resource-limited settings.
- Integration with Digital and Wearable Technologies: Combining EIA with digital sensors or wearable devices could provide real-time monitoring of biomarkers for personalized health management.
- Enhanced Automation and Robotics: Advanced robotics and automated systems could streamline EIA workflows, reduce human errors, and increase assay throughput.
- Enhanced Signal Detection Techniques: Developing more sensitive and efficient detection methods, such as advanced photonics and advanced imaging techniques, could improve signal detection in EIA.
- Quantum Dots and Upconversion Nanoparticles: These advanced nanoparticles offer unique optical properties, enabling multiplexing and higher sensitivity in EIA.
- Biosensors and Lab-on-a-Chip Platforms: Integrating EIA with biosensors and lab-on-a-chip platforms could lead to portable, rapid, and highly sensitive diagnostic devices.
- Artificial Intelligence (AI) and Data Analytics: AI algorithms and machine learning could enhance data analysis, improve signal-to-noise ratios, and aid in identifying complex patterns in large datasets.
- Customized and Personalized Assays: Tailoring EIA assays to individual patient profiles could lead to more accurate diagnoses and treatment plans in precision medicine.
- Bioinformatics Integration: Integrating EIA data with bioinformatics databases and resources could enhance the interpretation of results and contribute to a deeper understanding of biological processes.
- Enhanced Sample Preparation Techniques: Improved sample preparation methods could reduce interference and matrix effects, leading to more accurate and reliable results.
- Eco-Friendly and Sustainable Approaches: Developments in assay components and materials could lead to more environmentally friendly and sustainable EIA protocols.
FAQs:
What is Enzyme Immunoassay (EIA)?
Enzyme Immunoassay (EIA) is a laboratory technique that uses antibodies and enzymes to detect and quantify specific molecules, such as antigens or antibodies, in a sample. It relies on the specific binding of antigens and antibodies and the enzymatic reaction to generate a measurable signal.
How does EIA work?
EIA involves immobilizing one of the binding partners (antigen or antibody) onto a solid surface, such as a microtiter plate well. The sample containing the target molecule is added, and if the target is present, it binds to the immobilized partner. A labeled enzyme-conjugated antibody is then added, leading to an enzymatic reaction that generates a signal, which is proportional to the target’s concentration.
What are the different types of EIA?
EIA includes Direct ELISA, Indirect ELISA, Sandwich ELISA, Competitive ELISA, and more. Each type has a specific approach to utilizing antibodies and enzymes for detection, allowing flexibility in different applications.
What are the advantages of EIA?
EIA offers high sensitivity, specificity, and versatility. It can detect low concentrations of target molecules, quantitate them, and be applied in various fields, including clinical diagnostics, research, and quality control.
What are the limitations of EIA?
EIA can have limitations such as cross-reactivity, potential false results due to non-specific binding, sensitivity saturation at high concentrations, and interference from complex sample matrices.
What are the applications of EIA?
EIA has diverse applications, including clinical diagnostics (disease detection, hormone assays), pharmaceutical development, food safety, blood banking, virology, and environmental monitoring.
How is EIA different from ELISA?
EIA is a broader term encompassing all enzyme-based immunoassays, while ELISA specifically refers to the solid-phase immunoassays that use enzymes as labels for detection. ELISA is a type of EIA.
Can EIA be automated?
Yes, EIA can be automated using robotic systems and microplate readers, which allows high-throughput analysis of multiple samples and reduces human error.
What is the future of EIA?
The future of EIA involves advancements in nanotechnology, miniaturization, integration with digital technologies, enhanced signal detection methods, biosensors, AI integration, and more. These developments aim to improve sensitivity, speed, and applicability of EIA in various settings.
Conclusion:
In conclusion, Enzyme Immunoassay (EIA) is a powerful laboratory technique that utilizes antibodies and enzymes to detect and quantify specific molecules in various samples. With its high sensitivity, specificity, and adaptability, EIA finds widespread applications in clinical diagnostics, research, and industry. As technology continues to advance, EIA’s integration with emerging fields like nanotechnology, automation, and data analytics holds promise for even greater precision, efficiency, and innovation in molecular detection and analysis.
Possible References Used