Ehrlich’s Reagent is a chemical solution used primarily in laboratories for detecting the presence of certain compounds, particularly those with aromatic amino groups, such as tryptophan and other indoles. It is commonly used in clinical chemistry and research for various applications:
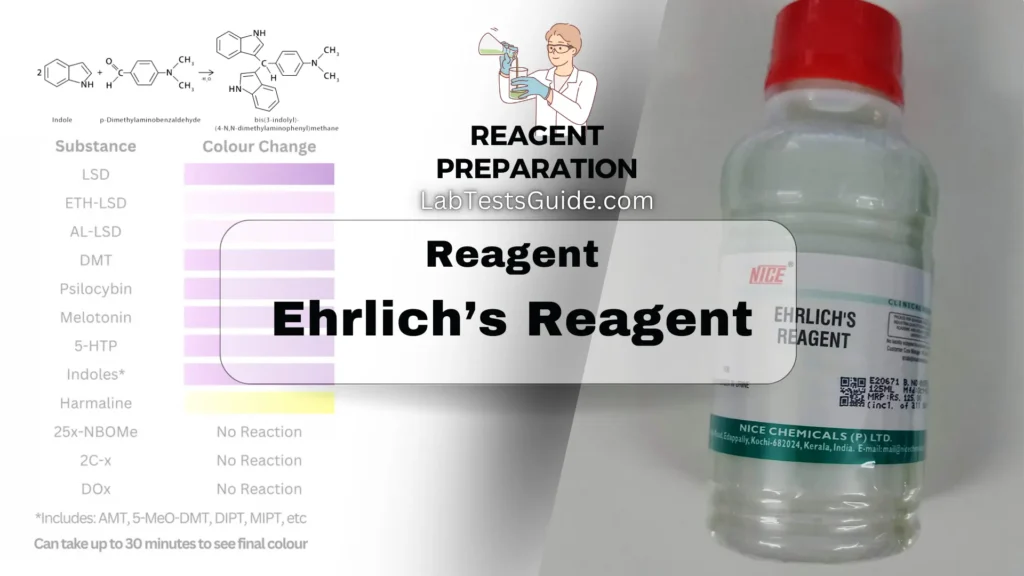
Uses of Ehrlich’s Reagent:
- Indole Detection: Identifies indole in urine, useful for diagnosing metabolic disorders like tryptophan metabolism issues.
- Tryptophan Detection: Helps detect tryptophan in biochemical research and clinical diagnostics.
- Bacterial Identification: Used in microbiology to identify indole-producing bacteria in culture tests.
- Drug Testing: Assists in detecting certain drugs and their metabolites in clinical and forensic labs.
- Biochemical Research: Employed to study compounds with aromatic amino groups in research settings.
Composition of Ehrlich’s Reagent:
| Component | Amount |
|---|---|
| 4-Dimethylaminobenzaldehyde | 4 g |
| Hydrochloric acid, concentrated | 40 mL |
| Distilled water | 160 mL |
Preparation of Ehrlich’s Reagent:
- Weigh 4 grams of 4-dimethylaminobenzaldehyde and transfer it to a leak-proof brown bottle.
- Measure 160 mL of distilled water and add it to the bottle containing the chemical. Mix until the 4-dimethylaminobenzaldehyde is dissolved.
- Add 40 mL of concentrated hydrochloric acid to the solution. Mix well.
- Caution: Concentrated hydrochloric acid is corrosive and produces injurious vapors. Handle it with care in a well-ventilated area or fume hood.
- Label the bottle clearly with “Corrosive” and store it at room temperature. The reagent is stable for several weeks if protected from daylight.
Precautions:
- Wear Protective Gear: Use gloves, safety goggles, and a lab coat to protect your skin and eyes from contact with chemicals.
- Use in a Well-Ventilated Area: Handle the reagent and concentrated hydrochloric acid in a fume hood or a well-ventilated room to avoid inhaling harmful vapors.
- Handle Concentrated Hydrochloric Acid with Care: It is highly corrosive. Avoid direct contact and ensure that the acid does not spill.
- Avoid Light Exposure: Store the prepared reagent in a dark, brown bottle to protect it from light, which can degrade the solution.
- Label Clearly: Mark the bottle with “Corrosive” to alert others of the chemical hazard.
- Proper Storage: Keep the reagent at room temperature and ensure it is sealed tightly to prevent contamination or degradation.
- Disposal: Dispose of any waste or leftover reagent according to your institution’s hazardous waste disposal guidelines.
Uses of Ehrlich’s Reagent in Clinical Laboratories:
- Indole Detection in Urine: To diagnose metabolic disorders and conditions affecting tryptophan metabolism by detecting the presence of indole in urine samples.
- Testing for Tryptophan: To identify tryptophan in biological fluids or tissues, aiding in nutritional and metabolic studies.
- Microbial Identification: To identify indole-producing bacteria in microbiological cultures, which can be crucial for diagnosing infections and determining bacterial species.
- Drug Metabolite Detection: To detect certain drugs and their metabolites in clinical and forensic testing.
- Biochemical Research: To study aromatic amino compounds and their metabolic products in research settings, providing insights into various biochemical processes.
Possible References Used