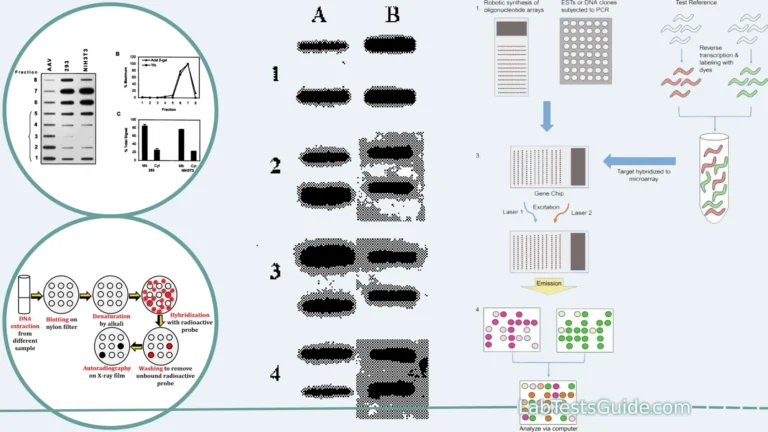
DNA sequencing is a laboratory technique used to determine the order of nucleotides (adenine, cytosine, guanine, and thymine) in a DNA molecule. It is a fundamental tool in molecular biology and genetics, with a wide range of applications in fields such as genomics, medicine, forensics, and evolutionary biology. There are several methods for DNA sequencing, but the most common approach is known as “Sanger sequencing” or “chain termination sequencing.

What is DNA Sequencing ?
DNA sequencing is a laboratory technique used to determine the precise order of nucleotides (A, C, G, T) in a DNA molecule. It reveals the genetic code and is essential for understanding genes, genetic variations, and numerous applications in genetics and biology.
Importance of DNA Sequencing:
The importance of DNA sequencing is vast and has far-reaching implications across various fields of science and medicine. Here is a list highlighting its significance:
- Understanding Genetics: DNA sequencing allows us to decode the genetic information stored in an organism’s DNA, providing insights into the structure and function of genes.
- Disease Diagnosis: It plays a crucial role in identifying genetic mutations responsible for inherited diseases and genetic disorders, aiding in early diagnosis and treatment.
- Cancer Research: DNA sequencing helps identify mutations in cancer cells, leading to personalized cancer treatments and a better understanding of tumor biology.
- Pharmacogenomics: It guides the development of personalized medicine by revealing how an individual’s genetic makeup influences their response to drugs.
- Evolutionary Biology: DNA sequencing helps trace evolutionary relationships among species, providing insights into the history of life on Earth.
- Agriculture and Crop Improvement: DNA sequencing is used to develop disease-resistant and genetically modified crops, enhancing food security.
- Forensic Science: It assists in criminal investigations by analyzing DNA evidence, identifying suspects, and confirming paternity.
- Microbiome Research: DNA sequencing helps characterize the microbial communities in various environments and their roles in health and disease.
- Biotechnology and Genetic Engineering: It enables the manipulation of genes for the production of biopharmaceuticals, biofuels, and other bioproducts.
- Conservation Biology: DNA sequencing aids in the identification of endangered species and the development of conservation strategies.
- Genetic Counseling: It provides valuable information to individuals and families about their genetic predispositions and potential health risks.
- Historical and Ancestral Studies: DNA sequencing allows people to trace their ancestry and uncover their genetic heritage.
- Disease Surveillance: It assists in tracking the spread of infectious diseases and monitoring the emergence of new pathogens.
- Genomic Research: DNA sequencing has been pivotal in numerous genome projects, such as the Human Genome Project, advancing our understanding of human genetics.
- Biomedical Research: It supports a wide range of studies investigating the genetic basis of various diseases, opening avenues for novel therapies and interventions.
- Environmental Science: DNA sequencing helps monitor biodiversity, detect environmental pollutants, and study the impact of climate change on ecosystems.
- Drug Discovery: Identifying genetic targets through sequencing accelerates the development of new drugs and therapies.
Applications of DNA Sequencing:
DNA sequencing has a wide range of applications across various fields of science and medicine. Here are some key applications of DNA sequencing:
- Genomic Research:
- Determining the complete sequence of an organism’s genome to understand its genetic makeup.
- Studying genetic variation among individuals and populations.
- Clinical Diagnostics:
- Identifying genetic mutations responsible for inherited diseases and disorders.
- Detecting somatic mutations in cancer to guide treatment decisions.
- Prenatal genetic testing to assess fetal health and identify genetic conditions.
- Pharmacogenomics:
- Personalizing drug treatments based on an individual’s genetic profile to optimize efficacy and minimize side effects.
- Cancer Genomics:
- Characterizing the genetic alterations in cancer cells to develop targeted therapies.
- Monitoring tumor evolution and drug resistance over time.
- Infectious Disease Genomics:
- Tracking the spread of infectious diseases, such as COVID-19, through genomic epidemiology.
- Identifying drug resistance mutations in pathogens like bacteria and viruses.
- Microbiome Analysis:
- Studying the composition and function of microbial communities in various environments, including the human gut, soil, and oceans.
- Evolutionary Biology:
- Tracing the evolutionary history of species by comparing their DNA sequences.
- Studying genetic adaptations and speciation events.
- Agriculture and Crop Improvement:
- Developing genetically modified crops with improved traits, such as resistance to pests or environmental stress.
- Ensuring the authenticity and quality of food products through DNA barcoding.
- Forensic Science:
- Analyzing DNA evidence to solve crimes, identify suspects, and confirm relationships (e.g., paternity testing).
- Biotechnology and Genetic Engineering:
- Engineering microorganisms for the production of biofuels, enzymes, and biopharmaceuticals.
- Creating genetically modified organisms with desired traits.
- Conservation Biology:
- Identifying and preserving endangered species by studying their DNA.
- Monitoring genetic diversity within populations to inform conservation strategies.
- Genetic Counseling:
- Providing individuals and families with information about their genetic predispositions and risks.
- Historical and Ancestral Studies:
- Tracing the genetic heritage and migration patterns of human populations.
- Identifying ancient human remains and archaeological artifacts through DNA analysis.
- Environmental Science:
- Assessing biodiversity and ecological health by analyzing environmental DNA (eDNA).
- Detecting and monitoring pollution and contaminants in natural ecosystems.
- Drug Discovery:
- Identifying potential drug targets and biomarkers through genomic and transcriptomic studies.
- Screening for genetic factors that influence drug response.
- Disease Surveillance:
- Monitoring the emergence and spread of infectious diseases by sequencing pathogens.
- Identifying potential zoonotic disease reservoirs in wildlife.
Principles of DNA Sequencing:
The principles of DNA sequencing involve deciphering the precise order of nucleotides (adenine, cytosine, guanine, and thymine) in a DNA molecule. While there are several sequencing methods, including newer techniques like next-generation and third-generation sequencing, the fundamental principles are often based on the original Sanger sequencing method. Here are the key principles of DNA sequencing:
- DNA Template: The DNA molecule to be sequenced serves as the template. This DNA may be genomic DNA, plasmid DNA, complementary DNA (cDNA), or any other source of interest.
- DNA Polymerase: A DNA polymerase enzyme is used to replicate the DNA template. DNA polymerases are responsible for adding complementary nucleotides to the growing DNA strand.
- DNA Primers: Short, single-stranded DNA sequences called primers are designed to anneal (bind) to specific regions of the DNA template. Primers provide a starting point for DNA synthesis.
- Nucleotides (dNTPs): Deoxyribonucleoside triphosphates (dNTPs), which are the building blocks of DNA, are provided in the reaction mixture. These include adenine (A), cytosine (C), guanine (G), and thymine (T).
- Dideoxynucleotides (ddNTPs): Dideoxynucleoside triphosphates (ddNTPs) are modified versions of dNTPs. Unlike dNTPs, they lack a 3′ hydroxyl group, which is required for the formation of the phosphodiester bond necessary for DNA chain elongation. Therefore, ddNTPs cause chain termination when incorporated into the growing DNA strand.
- Sequencing Reaction: The DNA sequencing reaction includes the DNA template, DNA polymerase, primers, dNTPs, and a mixture of ddNTPs (labeled with different fluorescent or radioactive tags). This reaction allows for DNA synthesis but leads to chain termination at positions where ddNTPs are incorporated.
- Chain Termination: During DNA synthesis, both regular dNTPs and ddNTPs are present in the reaction. When a ddNTP is added to the growing DNA strand, it lacks the 3′ hydroxyl group needed for further extension, causing the DNA synthesis process to terminate at that point.
- Gel Electrophoresis: After the sequencing reaction, the resulting mixture of DNA fragments is separated by size using gel electrophoresis. Smaller fragments move faster through the gel, while larger fragments move more slowly.
- Detection: The separated DNA fragments are detected using various methods, depending on the sequencing platform. In Sanger sequencing, this often involves fluorescence or autoradiography to visualize the positions of labeled ddNTPs along the gel.
- Data Analysis: The order of terminated fragments on the gel corresponds to the sequence of the original DNA template. Specialized software is used to analyze the detected signals and generate the DNA sequence.
These principles underlie both Sanger sequencing and many next-generation sequencing (NGS) techniques, although NGS methods have different approaches to generating sequence data. Understanding these principles is essential for researchers and technicians conducting DNA sequencing experiments and for interpreting the resulting sequence data accurately.
Sample Preparation:
Sample preparation is a crucial step in DNA sequencing and various molecular biology applications. It involves the collection, processing, and sometimes modification of biological samples to obtain high-quality DNA suitable for sequencing or other analyses. Proper sample preparation is essential for accurate and reliable results. Here are the key steps and considerations involved in sample preparation for DNA sequencing:
- Sample Collection: The type of biological sample you’re working with depends on your research goals. It can be DNA isolated from cells, tissues, blood, saliva, environmental samples, or ancient specimens like bones or hair.
- DNA Extraction: DNA must be extracted from the collected sample. This often involves breaking down cell walls or membranes and removing proteins, RNA, and other contaminants. Various DNA extraction kits and techniques are available, such as phenol-chloroform extraction, column-based purification, and magnetic bead-based methods.
- Quality Assessment: After extraction, it’s crucial to assess the quality and quantity of the isolated DNA. Common techniques for this include UV spectrophotometry, fluorometry, and gel electrophoresis. High-quality DNA should have minimal degradation and be relatively free from contaminants.
- DNA Quantification: Accurate quantification of DNA is essential to ensure you have sufficient material for downstream applications. Quantification can be done using various methods, including qPCR (quantitative polymerase chain reaction) or specialized fluorometric assays.
- DNA Fragmentation: Depending on the sequencing platform and application, DNA may need to be fragmented into smaller, manageable pieces. For example, next-generation sequencing (NGS) typically requires short DNA fragments, while long-read sequencing may require larger fragments.
- Library Preparation: In many sequencing applications, DNA libraries are created to facilitate sequencing. This involves adding specific adaptors or primers to the DNA fragments to enable their amplification and sequencing. Library preparation methods can vary based on the sequencing technology being used.
- Multiplexing: If you’re sequencing multiple samples, you may use barcoding or indexing during library preparation. This allows you to sequence multiple samples simultaneously, saving time and cost.
- Purification: To remove impurities, enzymes, and unincorporated nucleotides, DNA fragments or libraries are often purified using techniques such as bead-based cleanup or column-based purification.
- Quality Control: Throughout the sample preparation process, quality control steps should be implemented to ensure that the DNA fragments or libraries meet the desired specifications. This may include size selection, quantification, and assessing the absence of contaminants.
- Storage: Proper storage of DNA samples or libraries is essential to prevent degradation. DNA should be stored at low temperatures (typically -20°C or -80°C) to maintain its integrity until sequencing.
- Documentation: Detailed records should be maintained throughout the sample preparation process, including information on sample origin, extraction methods, quantification, and library preparation steps. This documentation is essential for traceability and reproducibility.
- Adherence to Protocols: Following standardized protocols and best practices for sample preparation is critical to obtaining reliable and reproducible results.
Sequencing Reaction:
The sequencing reaction, also known as the sequencing assay or sequencing reaction mixture, is a key step in DNA sequencing that involves the enzymatic synthesis of DNA fragments with the incorporation of chain-terminating nucleotides, which allows the determination of the sequence of the target DNA. This reaction is a fundamental part of the Sanger sequencing method, which was one of the earliest techniques used for DNA sequencing. Here’s an overview of the sequencing reaction:
Components of the Sequencing Reaction:
- DNA Template: The DNA template is the segment of DNA that you want to sequence. It serves as a template for the synthesis of a complementary DNA strand.
- Primer: A short, single-stranded DNA or RNA primer is used to initiate DNA synthesis. The primer anneals (binds) to a complementary sequence on the DNA template.
- DNA Polymerase: A DNA polymerase enzyme is essential for extending the primer and synthesizing a complementary DNA strand. DNA polymerases have the ability to add nucleotides to the growing DNA strand.
- Deoxyribonucleoside Triphosphates (dNTPs): The four standard dNTPs—adenine (A), cytosine (C), guanine (G), and thymine (T)—are the building blocks used to synthesize the new DNA strand. They provide the necessary nucleotides for base pairing with the template.
- Dideoxyribonucleoside Triphosphates (ddNTPs): These are modified versions of dNTPs that lack a 3′ hydroxyl group. They are labeled with fluorescent or radioactive tags specific to each base (A, C, G, and T). Importantly, ddNTPs cause chain termination when they are incorporated into the growing DNA strand because they cannot form the next phosphodiester bond needed for elongation.
Sequencing Reaction Process:
- The sequencing reaction is set up in multiple reaction tubes, each containing the same components (template, primer, DNA polymerase, dNTPs), except for one of the four ddNTPs (A, C, G, or T).
- DNA synthesis begins as the primer anneals to the DNA template.
- As the DNA polymerase extends the primer along the template, it incorporates standard dNTPs complementary to the template sequence.
- Occasionally, a ddNTP, specific to one of the four bases, is incorporated instead of the corresponding dNTP. This causes chain termination at that position because ddNTPs lack the 3′ hydroxyl group necessary for the formation of the next phosphodiester bond.
- The result is a collection of DNA fragments of varying lengths, each terminating with a ddNTP at different positions along the template.
Analysis and Visualization:
After the sequencing reaction, the mixture of terminated DNA fragments is typically analyzed using gel electrophoresis or capillary electrophoresis. The fragments are separated by size, forming a pattern known as a sequencing ladder. The position of each terminated fragment in the ladder corresponds to the base that was incorporated (A, C, G, or T) and, therefore, reveals the sequence of the original DNA template.
Modern DNA sequencers automate this process, allowing for high-throughput and efficient DNA sequencing. Sanger sequencing was foundational in genomics but has been largely supplanted by next-generation sequencing (NGS) technologies, which can sequence millions of DNA fragments in parallel, enabling rapid and cost-effective genome analysis. However, Sanger sequencing is still used for specific applications and quality control purposes in molecular biology laboratories.
Chain Termination:
Chain termination is a critical concept in DNA sequencing, particularly in the Sanger sequencing method. It refers to the process by which DNA synthesis is halted or “terminated” at specific positions along the DNA strand during sequencing. Chain termination occurs when a modified nucleotide, called a dideoxynucleotide (ddNTP), is incorporated into the growing DNA strand instead of a regular deoxyribonucleotide (dNTP). Here’s a more detailed explanation of chain termination in DNA sequencing:
Key Points:
- Standard Deoxyribonucleotides (dNTPs): In DNA replication and synthesis, DNA polymerase incorporates standard deoxyribonucleotides (dNTPs) into the growing DNA strand. These dNTPs contain the four DNA bases: adenine (A), cytosine (C), guanine (G), and thymine (T).
- Dideoxyribonucleotides (ddNTPs): Dideoxyribonucleotides (ddNTPs) are modified versions of dNTPs. Unlike dNTPs, ddNTPs lack a 3′ hydroxyl group (-OH) on the sugar moiety. This missing hydroxyl group prevents the formation of the phosphodiester bond necessary for DNA chain elongation.
- Chain Termination: During DNA sequencing, both dNTPs and ddNTPs are present in the sequencing reaction mixture. When a ddNTP is incorporated into the growing DNA strand, it acts as a “terminator” because it cannot serve as a substrate for further DNA synthesis.
- Random Termination: Since the sequencing reaction includes all four dNTPs and one of the four ddNTPs (labeled with a specific fluorescent or radioactive marker), DNA synthesis can randomly terminate at positions where a ddNTP is added. This occurs at varying positions along the DNA template, resulting in a collection of terminated DNA fragments of different lengths.
- Sequencing Ladder: After electrophoresis or capillary electrophoresis, the terminated DNA fragments are separated by size, forming a pattern called a sequencing ladder. Each band in the ladder corresponds to a specific position where DNA synthesis was terminated by the incorporation of a ddNTP.
- Base Identification: By examining the sequencing ladder and noting the position of each terminated fragment, it is possible to deduce the sequence of the original DNA template. The ddNTP-specific label associated with each ddNTP (A, C, G, or T) helps identify the corresponding base at each termination point.
Practical Significance:
Chain termination is the basis for the Sanger sequencing method, which was one of the earliest techniques for DNA sequencing. This method allowed researchers to determine the precise sequence of DNA fragments and played a pivotal role in deciphering the human genome and understanding the genetic code. While newer sequencing technologies like next-generation sequencing (NGS) have largely supplanted Sanger sequencing for large-scale genomic projects due to their higher throughput, Sanger sequencing is still used for specific applications, such as sequencing individual DNA fragments or verifying sequences obtained from NGS.
Electrophoresis:
Electrophoresis is a laboratory technique used to separate and analyze molecules based on their size, charge, or other physical properties in an electric field. It is widely employed in molecular biology, biochemistry, and genetics for various applications, including DNA sequencing, protein analysis, and the separation of macromolecules. There are several types of electrophoresis, but the principles are generally consistent:
Basic Principles of Electrophoresis:
- Electric Field: Electrophoresis relies on the application of an electric field within a gel or liquid medium. This electric field is created by applying a voltage across the gel or buffer solution, causing charged molecules to move in response to the field.
- Gel Matrix: Electrophoresis is typically performed within a gel matrix, which can be made of agarose or polyacrylamide for nucleic acid analysis or polyacrylamide for protein analysis. The gel acts as a sieve, impeding the movement of molecules through it based on their size and charge.
- Sample Loading: The molecules to be separated are loaded onto the gel, often in wells or slots created on one end of the gel. These samples can include DNA fragments, RNA, proteins, or other molecules of interest.
- Migration of Molecules: When the electric field is applied, charged molecules within the gel will migrate through the matrix at different rates. Smaller molecules or molecules with a higher charge-to-mass ratio will move more quickly, while larger or less charged molecules will move more slowly.
- Separation: As the molecules move through the gel, they become separated into discrete bands or zones based on their size or charge. Smaller molecules travel farther from the loading point, while larger molecules remain closer to the starting point.
- Visualization: After electrophoresis, the separated molecules are typically visualized using various techniques. This can include staining the molecules with dyes that bind specifically to nucleic acids or proteins. In the case of DNA, ethidium bromide or specialized DNA stains are often used.
- Analysis: The separated molecules’ positions within the gel can be analyzed to determine their sizes or other properties. This information is valuable for various applications, such as DNA fragment sizing, protein purity assessment, or identifying specific molecules in a mixture.
Types of Electrophoresis:
- Agarose Gel Electrophoresis: Used for separating nucleic acids (DNA or RNA) based on their size. Smaller fragments migrate faster through the gel, while larger fragments move more slowly.
- Polyacrylamide Gel Electrophoresis (PAGE): Employed for higher-resolution separation of proteins based on size or charge. It is commonly used in protein analysis and can be denaturing or native, depending on the goals of the experiment.
- Capillary Electrophoresis (CE): A highly automated and high-throughput technique that separates molecules in a narrow capillary tube filled with a buffer. CE is used for DNA sequencing, DNA fragment analysis, and protein analysis.
- Isoelectric Focusing (IEF): Separates proteins based on their isoelectric points, which is the pH at which they have no net charge. In IEF, proteins migrate to their isoelectric point and stop moving.
- Two-Dimensional Electrophoresis (2DE): Combines two electrophoretic separations, typically isoelectric focusing followed by SDS-PAGE, to achieve high-resolution separation of complex mixtures of proteins.
- Pulsed-Field Gel Electrophoresis (PFGE): Used for resolving very large DNA fragments, such as whole chromosomes. It employs alternating electric fields to achieve separation.
- RNA Electrophoresis: A specialized form of electrophoresis used to separate and analyze RNA molecules based on size, integrity, or modifications.
Electrophoresis is a versatile and widely used technique in molecular biology and biochemistry, allowing scientists to separate and analyze a wide range of molecules for various research and diagnostic purposes.
Detection Methods:
There have been significant advancements in DNA sequencing technologies over the years, leading to various detection methods. Here are some key DNA sequencing detection methods:
- Sanger Sequencing:
- Chain Termination Method: In Sanger sequencing, chain termination is used to detect the sequence. As DNA polymerase incorporates dideoxynucleotides (ddNTPs), which lack a 3′ hydroxyl group, into the growing DNA strand, synthesis terminates at different positions along the template. These terminated fragments are separated by size through gel electrophoresis, and the sequence is determined by analyzing the pattern of terminated fragments.
- Next-Generation Sequencing (NGS):
- Fluorescent Labeling: NGS platforms use reversible terminators labeled with different fluorescent tags for each base (A, C, G, T). As each base is incorporated into the growing DNA strand, a fluorescent signal is recorded, allowing for real-time detection of the sequence.
- Pyrosequencing: Measures the release of pyrophosphate (PPi) during DNA synthesis. When a nucleotide is incorporated into the growing strand, PPi is released, triggering a series of enzymatic reactions that produce light. The emitted light is detected and used to identify the base.
- Ion Semiconductor Sequencing: Detects changes in pH as hydrogen ions are released when nucleotides are incorporated into the strand. pH changes are converted into electrical signals, enabling base identification.
- Nanopore Sequencing: Utilizes a protein pore embedded in a membrane. As DNA passes through the pore, individual bases disrupt the ionic current, allowing for direct sequencing of the DNA molecule.
- Third-Generation Sequencing:
- PacBio Sequencing (Single-Molecule Real-Time or SMRT Sequencing): Measures the time it takes for DNA polymerase to incorporate each nucleotide into the growing strand. Different fluorescent labels are used for each base, allowing real-time detection.
- Oxford Nanopore Sequencing: Similar to nanopore sequencing in NGS but uses a nanopore protein to directly read the DNA sequence as it passes through the pore.
- Mass Spectrometry:
- Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS): Fragments DNA into smaller pieces and then analyzes the mass-to-charge ratios of these fragments to determine the sequence.
- Pyrosequencing:
- Pyrophosphate Detection: Measures the release of pyrophosphate (PPi) during DNA synthesis. PPi release is proportional to the number of nucleotides incorporated, allowing for sequencing.
- Sequencing by Hybridization:
- Microarray-Based Sequencing: Utilizes microarrays containing known DNA probes that hybridize with the DNA template. The hybridization pattern reveals the sequence of the template.
- Sequencing by Ligation:
- Polony Sequencing: Involves attaching a DNA template to a solid support and performing repeated cycles of ligation with fluorescently labeled probes, revealing the sequence.
- Nanopore Sequencing:
- Direct Electrical Detection: Detects changes in electrical current as DNA molecules pass through a nanopore. The sequence is determined by the distinctive current disruptions caused by each base.
Data Analysis:
Data analysis is the process of inspecting, cleaning, transforming, and interpreting data to discover meaningful insights, patterns, and trends. In the context of DNA sequencing and genomics, data analysis is crucial for extracting biological information from the vast amount of raw sequencing data generated by modern sequencing technologies. Here are the key steps and considerations in DNA sequencing data analysis:
- Data Preprocessing:
- Quality Control: Assess the quality of sequencing data, including factors like base quality scores, read length, and overall data integrity. Low-quality data may need to be filtered or trimmed.
- Adapter and Contaminant Removal: Remove any sequencing adapters or contaminating sequences introduced during library preparation.
- Read Alignment: Map sequencing reads to a reference genome or transcriptome for further analysis. Different alignment algorithms are used based on the type of data (e.g., DNA, RNA, whole-genome, targeted sequencing).
- Variant Calling:
- SNP and Indel Detection: Identify single nucleotide polymorphisms (SNPs) and small insertions/deletions (indels) by comparing aligned reads to the reference sequence.
- Structural Variant Detection: Detect larger genomic alterations, such as insertions, deletions, duplications, inversions, and translocations.
- Annotation:
- Functional Annotation: Annotate variants with information about their potential functional effects, such as whether they affect protein-coding genes, regulatory elements, or non-coding RNA.
- Population Frequency: Determine the frequency of identified variants in different populations using databases like dbSNP and gnomAD.
- Variant Filtering and Prioritization:
- Filtering Criteria: Apply filters to retain only high-confidence variants based on factors like read depth, allele frequency, and quality scores.
- Pathogenicity Prediction: Predict the potential clinical significance of variants using computational tools and databases.
- Variant Interpretation:
- Clinical Significance: Assess the clinical relevance of variants, particularly in the context of genetic diseases.
- Genotype-Phenotype Associations: Investigate associations between identified variants and observed phenotypes.
- Functional Analysis:
- Gene Expression Analysis: Analyze RNA sequencing data to measure gene expression levels and identify differentially expressed genes.
- Pathway and Functional Enrichment Analysis: Determine which biological pathways and gene functions are affected by differentially expressed genes or variants.
- Comparative Genomics:
- Comparative Analysis: Compare the genomes or transcriptomes of different individuals or species to identify conserved regions, divergent sequences, or evolutionary changes.
- Visualization:
- Data Visualization: Create plots, graphs, and visual representations to help interpret and communicate the results effectively.
- Genome Browsers: Utilize genome browsers and visualization tools to explore genomic data in the context of reference genomes and annotations.
- Statistical Analysis:
- Statistical Tests: Apply appropriate statistical tests to assess the significance of observed differences or associations in the data.
- Multiple Testing Correction: Account for multiple comparisons to control the false discovery rate.
- Report Generation:
- Result Summarization: Summarize key findings, including identified variants, their annotations, and relevant statistics.
- Clinical Reports: Generate reports with clinically relevant information for healthcare professionals in diagnostic or research settings.
- Data Storage and Sharing:
- Data Management: Organize and store sequencing data, analysis pipelines, and results in a structured and secure manner.
- Data Sharing: Share data and results with collaborators, researchers, or the broader scientific community through appropriate repositories and databases.
- Continuous Updating:
- Stay Informed: Keep up with the latest advancements in sequencing technologies and analysis tools to refine and improve analysis methods.
Future Directions:
The field of DNA sequencing and genomics is constantly evolving, and there are several exciting future directions and trends that are likely to shape the field in the coming years. Here are some key areas of interest and potential developments:
- Long-Read Sequencing Technologies: The continued development of long-read sequencing technologies, such as Oxford Nanopore and Pacific Biosciences, is expected to improve the sequencing of complex regions of the genome, facilitate de novo assembly of genomes, and better characterize structural variations.
- Single-Cell Sequencing: Single-cell sequencing techniques will become more widespread, enabling the study of cellular heterogeneity within tissues and complex biological systems. This will have applications in fields like cancer research, neuroscience, and developmental biology.
- Epigenomics: Epigenomic sequencing, which investigates modifications to DNA and histones, will provide deeper insights into gene regulation, chromatin structure, and the role of epigenetics in health and disease.
- Functional Genomics: Integration of genomics with functional assays like CRISPR-Cas9 screens and transcriptomics will enhance our understanding of gene function and regulatory networks.
- Metagenomics: Advances in metagenomic sequencing will enable a more comprehensive characterization of complex microbial communities, aiding research in environmental microbiology, human microbiome studies, and infectious disease surveillance.
- Personalized Genomic Medicine: The incorporation of genomic information into clinical practice will continue to expand, allowing for more precise diagnosis, treatment selection, and disease risk prediction.
- Artificial Intelligence (AI) and Machine Learning: AI and machine learning algorithms will play an increasingly important role in DNA sequence analysis, aiding in variant interpretation, functional predictions, and data integration.
- Ethical and Privacy Considerations: As genomics becomes more integrated into healthcare and research, addressing ethical and privacy concerns related to data sharing, informed consent, and genomic discrimination will be critical.
- Environmental Genomics: Environmental DNA (eDNA) sequencing will be used to monitor and assess biodiversity, track invasive species, and study ecosystem dynamics.
- Rare Disease Genomics: Genomic research will continue to uncover the genetic underpinnings of rare and undiagnosed diseases, potentially leading to new treatments and therapies.
- Cancer Genomics: Advancements in cancer genomics will further elucidate the genetic basis of cancer, leading to improved diagnostics, targeted therapies, and precision medicine approaches.
- Nanopore Sequencing Innovations: Further innovations in nanopore sequencing technologies, such as improved accuracy and read lengths, may make it more competitive with short-read sequencing methods.
- Innovations in Data Storage and Analysis: Scalable and efficient solutions for storing, managing, and analyzing vast genomic datasets will be essential to handle the increasing volume of genomic data generated.
- Environmental and Social Impact Assessment: Research assessing the environmental and societal impacts of genomics, such as the ethical implications of gene editing and the ecological consequences of gene drives, will continue to be important.
- Global Collaboration: Collaborative efforts and data sharing across international boundaries will accelerate genomics research and its applications.
FAQs:
What is DNA sequencing?
Answer: DNA sequencing is the process of determining the precise order of nucleotides (A, C, G, and T) in a DNA molecule. It is essential for understanding genetic information and has various applications in genetics, genomics, and biology.
Why is DNA sequencing important?
Answer: DNA sequencing is crucial for studying genetic variation, identifying disease-causing mutations, understanding evolutionary relationships, and advancing personalized medicine. It has revolutionized genetics research and clinical diagnostics.
What are the different DNA sequencing methods?
Answer: There are several DNA sequencing methods, including Sanger sequencing (first-generation), next-generation sequencing (NGS), and third-generation sequencing (e.g., PacBio and Oxford Nanopore). Each method has its own strengths and applications.
How does NGS work?
Answer: NGS involves the parallel sequencing of millions of DNA fragments. These fragments are sequenced simultaneously, and bioinformatics tools are used to assemble and analyze the data. NGS is faster and more cost-effective than traditional Sanger sequencing.
What is the Human Genome Project?
Answer: The Human Genome Project was an international research effort that successfully mapped and sequenced the entire human genome. It provided valuable insights into human genetics and served as a foundation for subsequent genomics research.
What are the applications of DNA sequencing?
Answer: DNA sequencing has diverse applications, including genome sequencing, genetic disease diagnosis, cancer genomics, evolutionary studies, forensics, and understanding microbial communities (metagenomics).
What is the difference between genomics and genetics?
Answer: Genetics focuses on the study of individual genes and their inheritance patterns, while genomics is the broader study of an organism’s entire genome, including all of its genes and their interactions.
What is personalized medicine?
Answer: Personalized medicine tailors medical treatment and healthcare decisions to an individual’s genetic and molecular characteristics. DNA sequencing is a key component of personalized medicine, helping to identify the most effective treatments for patients.
How is DNA sequencing used in forensics?
Answer: DNA sequencing is used in forensics to analyze biological evidence, such as blood or hair samples, to identify individuals or link suspects to crime scenes. DNA profiles are compared to databases for identification.
What are some ethical considerations in genomics research?
Answer: Ethical considerations in genomics include issues related to privacy, informed consent, genetic discrimination, the potential misuse of genetic information, and the responsible use of gene-editing technologies like CRISPR-Cas9.
Conclusion:
In conclusion, DNA sequencing is a transformative technology that has revolutionized genetics and genomics research. From the early days of Sanger sequencing to the advent of next-generation and third-generation sequencing technologies, our ability to decode the genetic code has advanced significantly. DNA sequencing plays a pivotal role in diverse fields, including medicine, biology, forensics, and environmental science. As the field continues to evolve, it holds the promise of unlocking further insights into the complexities of life, health, and disease, ultimately benefiting humanity in numerous ways.
Home | Blog | About Us | Contact Us | Disclaimer
Possible References Used