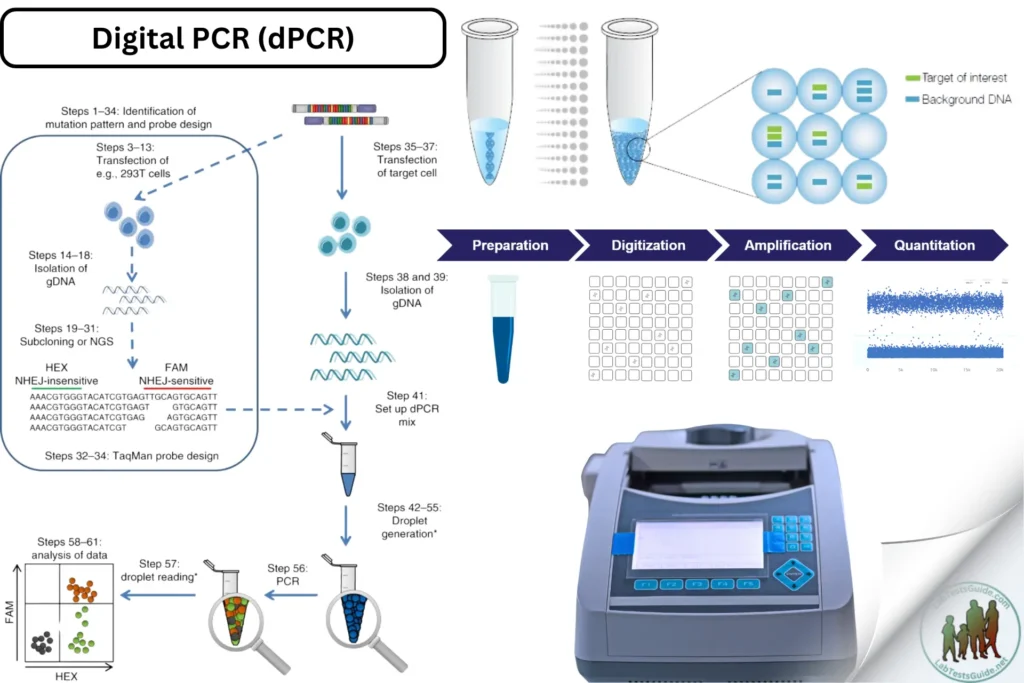
Digital PCR (dPCR) is an advanced technique for the absolute quantification of nucleic acids. Unlike traditional PCR, which provides relative quantification, dPCR divides the sample into many small partitions, performing PCR in each partition to count the number of DNA molecules directly. It is used for applications such as rare mutation detection, copy number variation analysis, and precise quantification of viral load.
Methodology for Digital PCR (dPCR) in Clinical Laboratory
Introduction
Digital PCR (dPCR) is an advanced technique for the absolute quantification of nucleic acids. Unlike traditional PCR, which provides relative quantification, dPCR divides the sample into many small partitions, performing PCR in each partition to count the number of DNA molecules directly. It is used for applications such as rare mutation detection, copy number variation analysis, and precise quantification of viral load.
Principle
The sample is partitioned into thousands of individual reactions. Each partition contains zero, one, or more target DNA molecules. After amplification, partitions are analyzed to determine the presence (positive) or absence (negative) of the target sequence, allowing for absolute quantification by counting positive partitions.
Specimen Requirements
- Type of specimen: Blood, plasma, serum, tissue samples, swabs, or other biological fluids
- Volume of specimen required: Typically 5-50 µL
- Collection method: Standard clinical procedures for sample collection (e.g., venipuncture, swabs)
- Handling and storage requirements: Store samples at -80°C for long-term storage; avoid repeated freeze-thaw cycles
- Stability of the specimen: DNA is generally stable, but follow specific guidelines for sample type
Reagents and Materials
- DNA extraction kit
- dPCR master mix
- Specific primers and probes for the target DNA
- Partitioning oil or chips (depending on the dPCR platform)
- Nuclease-free water
- Positive and negative control DNA
Equipment
- dPCR instrument (e.g., Bio-Rad QX200, Thermo Fisher QuantStudio 3D)
- Thermal cycler
- Microcentrifuge
- Vortex mixer
- Micropipettes and RNase/DNase-free tips
- dPCR consumables (chips, cartridges, or plates depending on the system)
- Biosafety cabinet (for handling DNA)
Procedure
DNA Extraction:
- Extract DNA from the sample using a DNA extraction kit following the manufacturer’s instructions.
- Quantify the extracted DNA using a spectrophotometer or fluorometer.
- Assess DNA quality and integrity using gel electrophoresis or a bioanalyzer (optional).
Partitioning:
- Prepare the dPCR reaction mix:
- DNA template: 1-10 ng
- dPCR master mix: 1X
- Forward and reverse primers: 0.2-0.5 µM each
- Probe (if using): 0.1-0.2 µM
- Nuclease-free water to the final volume (usually 20-25 µL)
- Load the reaction mix into the dPCR partitioning device (chips, plates, or droplets depending on the system).
- Partition the sample into individual reactions using the dPCR instrument.
PCR Amplification:
- Set up the PCR cycling conditions:
- Initial denaturation: 95°C for 10 minutes
- Amplification (40-45 cycles):
- Denaturation: 95°C for 30 seconds
- Annealing/Extension: 55-60°C for 1-2 minutes
- Final extension: 72°C for 5 minutes (if required)
- Perform PCR amplification in the thermal cycler.
Detection and Quantification:
- After amplification, load the partitions into the dPCR instrument for fluorescence detection.
- Analyze the partitions to determine the number of positive (fluorescent) and negative (non-fluorescent) partitions.
- Calculate the absolute quantity of target DNA using Poisson statistics based on the proportion of positive partitions.
Quality Control
- No-Template Controls (NTCs): Include NTCs to check for contamination.
- Positive and Negative Controls: Validate the efficiency and specificity of the assay.
- Partitioning Efficiency: Ensure proper partitioning of the sample to avoid false negatives or positives.
- Instrument Calibration: Regularly calibrate the dPCR instrument according to the manufacturer’s guidelines.
Calculation and Interpretation
- Absolute Quantification: Calculate the absolute number of target DNA molecules in the sample based on the number of positive partitions and Poisson statistics.
- Result Interpretation: Compare results with established reference ranges or clinical guidelines for accurate interpretation.
- Data Analysis Software: Use the manufacturer’s software for data analysis and visualization.
Limitations
- Sample Quality: Poor-quality DNA can affect partitioning and amplification efficiency.
- Complexity: dPCR is more complex and time-consuming compared to traditional PCR.
- Cost: dPCR requires specialized equipment and reagents, making it more expensive.
Safety Precautions
- PPE: Use appropriate PPE (gloves, lab coat, safety goggles) when handling samples and reagents.
- Clean Environment: Work in a DNase/RNase-free environment to prevent contamination.
- Waste Disposal: Dispose of waste according to local regulations and institutional guidelines.
References
- Huggett, J. F., Foy, C. A., Benes, V., Emslie, K., Garson, J. A., Haynes, R., … & Saunders, N. A. (2013). The digital MIQE guidelines: Minimum Information for Publication of Quantitative Digital PCR Experiments. Clinical Chemistry, 59(6), 892-902.
- dPCR instrument manufacturer’s guidelines and protocols.
- Molecular Cloning: A Laboratory Manual (Fourth Edition) by Michael R. Green and Joseph Sambrook.
- Clinical guidelines and standards from institutions such as the American Society for Microbiology (ASM) and the Clinical and Laboratory Standards Institute (CLSI).
This format provides a structured approach to conducting dPCR in clinical laboratories, focusing on accurate absolute quantification of nucleic acids, quality control, and safety precautions for reliable results.
Possible References Used