Colorimetric assays are analytical techniques used in chemistry and biochemistry to determine the concentration of a substance in a sample by measuring the intensity of color produced when a specific chemical reaction occurs. These assays are widely used in various fields, including clinical diagnostics, environmental monitoring, food testing, and research laboratories. Colorimetric assays are popular due to their simplicity, cost-effectiveness, and speed.
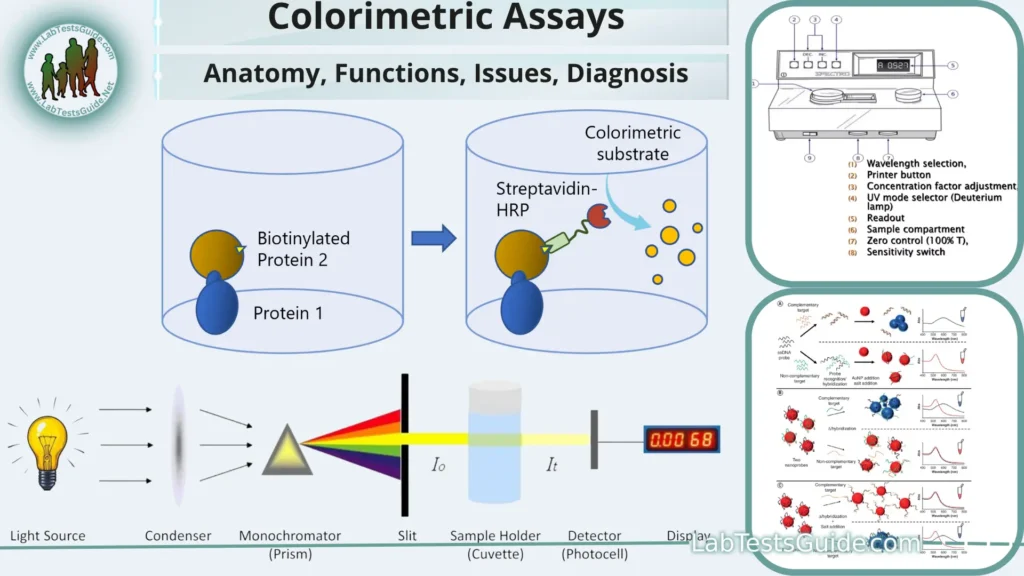
Defination of Colorimetric Assays:
Colorimetric assays are analytical techniques that measure the concentration of a substance in a sample by detecting and quantifying changes in color produced during a chemical reaction specific to the analyte of interest.
Importance of Colorimetric Assays:
The importance of colorimetric assays lies in their wide range of applications across various fields, making them a valuable tool in analytical chemistry and biology. Here are some key reasons why colorimetric assays are important:
- Simplicity and Accessibility: Colorimetric assays are relatively simple to perform and do not require expensive equipment or specialized training. This accessibility makes them suitable for a wide range of users, from researchers to clinicians and technicians.
- Cost-Effective: Compared to some other analytical techniques, colorimetric assays are often cost-effective, making them an attractive option for laboratories with budget constraints.
- Rapid Results: Colorimetric assays can provide results quickly, which is crucial in fields like clinical diagnostics, where timely information is essential for patient care.
- High Sensitivity: Some colorimetric assays can achieve high levels of sensitivity, allowing for the detection of trace amounts of analytes, such as specific biomarkers in clinical samples or contaminants in environmental samples.
- Quantitative Analysis: Colorimetric assays can provide quantitative data, allowing for precise measurement of analyte concentrations.
- Versatility: They can be applied to a wide range of analytes, including ions, small molecules, proteins, nucleic acids, and more. This versatility makes them valuable in diverse areas such as environmental monitoring, food safety testing, and medical diagnostics.
- Automation: Many colorimetric assays can be automated, increasing throughput and reducing the potential for human error.
- Research and Discovery: In scientific research, colorimetric assays are used to investigate biochemical and chemical processes, aiding in the discovery of new compounds, understanding biological mechanisms, and conducting drug screening.
- Quality Control: Colorimetric assays are utilized in quality control processes in industries like pharmaceuticals and food production to ensure product consistency and safety.
- Teaching and Education: They are valuable tools in educational settings for teaching students fundamental principles of chemistry, biochemistry, and analytical techniques.
- Environmental Monitoring: Colorimetric assays are employed to detect pollutants and contaminants in environmental samples, contributing to the assessment of water and air quality.
- Clinical Diagnostics: Colorimetric assays play a critical role in diagnosing various medical conditions by detecting biomarkers, hormones, enzymes, and other analytes in patient samples.
Applications of Colorimetric Assays:
Colorimetric assays find applications in various fields due to their versatility, simplicity, and cost-effectiveness. Here are some common applications of colorimetric assays:
- Clinical Diagnostics:
- Enzyme-Linked Immunosorbent Assay (ELISA): Used to detect antibodies, antigens, hormones, and various biomarkers in patient samples for disease diagnosis.
- Blood Glucose Monitoring: Colorimetric assays are used in glucose test strips for diabetic patients to measure blood glucose levels.
- Environmental Monitoring:
- Water Quality Analysis: Colorimetric assays are employed to detect pollutants like heavy metals, nitrates, and phosphates in water samples.
- Air Quality Monitoring: They can be used to measure air pollutants like ozone, nitrogen dioxide, and particulate matter.
- Food and Beverage Industry:
- Food Safety Testing: Colorimetric assays are used to detect pathogens, allergens, and contaminants in food products.
- Nutrient Analysis: Used to determine the concentration of nutrients like vitamins and minerals in food and beverages.
- Pharmaceuticals:
- Drug Assays: Colorimetric assays are utilized in drug development and quality control to assess the purity and concentration of pharmaceutical compounds.
- Drug Screening: They can be employed in high-throughput drug screening assays to identify potential drug candidates.
- Biotechnology and Molecular Biology:
- Nucleic Acid Quantification: Colorimetric assays like the Bradford assay are used to quantify DNA and RNA concentrations.
- Protein Assays: Employed for protein quantification and characterization.
- Research and Education:
- Biochemical Studies: Colorimetric assays are fundamental in elucidating biochemical processes and mechanisms.
- Teaching Tools: Used in educational settings to teach students about analytical techniques and chemical reactions.
- Veterinary Medicine:
- Animal Health: Colorimetric assays are applied to diagnose diseases and monitor the health of animals by measuring specific biomarkers.
- Industrial Processes:
- Quality Control: Used to ensure product quality and consistency in industries such as textiles, chemicals, and cosmetics.
- Agriculture:
- Soil Analysis: Colorimetric assays help assess soil nutrient content and pH levels for optimizing crop growth.
- Forensics:
- Blood and Urine Analysis: Applied in forensic laboratories to analyze biological samples for the presence of drugs, toxins, or other substances.
- Art and Conservation:
- Art Restoration: Colorimetric assays are used to assess the deterioration of artworks and historical artifacts.
- Oil and Petrochemical Industry:
- Oil Quality Analysis: Colorimetric assays can be employed to determine oil quality, including measuring impurities and degradation products.
- Chemical Research:
- Chemical Analysis: They are used in various chemical analyses to determine the concentration of specific compounds.
Principles of Colorimetry:
Colorimetry is a branch of analytical chemistry that deals with the quantitative determination of the concentration of a substance by measuring the absorbance or transmittance of light by a colored solution. The principles of colorimetry are based on the interaction between light and matter, particularly the absorption of specific wavelengths of light by substances in solution. Here are the fundamental principles of colorimetry:
- Absorption of Light: When light passes through a solution, some wavelengths of light may be absorbed by the solute (the substance dissolved in the solution). This absorption occurs when the energy of the photons in the light matches the energy required to promote electrons in the solute from lower to higher energy states. As a result, certain colors or wavelengths are selectively absorbed.
- Beer-Lambert Law: The Beer-Lambert law describes the relationship between the concentration of a solute in a solution and the absorbance of light by that solution. It is expressed as:A = ε * b * cWhere:
- A is the absorbance of the solution.
- ε (epsilon) is the molar absorptivity or molar absorptivity coefficient, which is a constant specific to the substance and the wavelength of light.
- b is the path length through which the light passes in the sample (typically in centimeters).
- c is the concentration of the solute in the solution (typically in molar or other appropriate units).
- Selecting the Wavelength: To perform a colorimetric assay, it’s crucial to choose an appropriate wavelength of light that corresponds to the absorption maximum (peak) of the substance being analyzed. This ensures that the absorbance measurement is most sensitive to changes in concentration.
- Standard Curve: To quantify the concentration of an unknown sample, a standard curve is constructed. This curve is created by measuring the absorbance of standard solutions with known concentrations of the analyte at the selected wavelength. By comparing the absorbance of the unknown sample to the standard curve, the concentration of the analyte in the unknown sample can be determined.
- Limitations: Colorimetry is most suitable for substances that exhibit color due to their selective absorption of light. Colorless substances or those with weak absorption may require more sensitive techniques like UV-Visible spectrophotometry.
- Instrumentation: Colorimeters and spectrophotometers are common instruments used in colorimetry. They emit a specific wavelength of light through the sample, and a detector measures the amount of light absorbed or transmitted. The resulting data are used to calculate the absorbance.
List of Colorimetric Assays:
Colorimetric assays are versatile and widely used in various fields for quantitative analysis. Here is a list of some common colorimetric assays, along with their applications:
- Bradford Assay: Used for protein quantification by measuring the absorbance shift of Coomassie Brilliant Blue dye.
- Benedict’s Test: Detects reducing sugars, including glucose, by producing a color change from blue to red-brown.
- Folin-Ciocalteu Assay: Measures the total phenolic content in samples, often applied in food and plant research.
- Lowry Assay: Quantifies protein concentration by reacting with copper ions and Folin-Ciocalteu reagent, leading to a color change.
- Biuret Test: Used for protein concentration estimation, based on the formation of a violet color complex with copper ions.
- Bicinchoninic Acid (BCA) Assay: Measures protein concentration by forming a purple-colored complex with copper ions.
- Ninhydrin Test: Detects amino acids and peptides by producing a blue or purple color when reacting with free amino groups.
- Orcinol Assay: Measures the concentration of reducing sugars and pentoses, leading to a color change from yellow to orange-red.
- Nessler’s Reagent: Detects the presence of ammonia ions in water samples, resulting in a yellow to brown color change.
- Phenolphthalein Alkalinity Test: Determines the alkalinity of water samples by monitoring a pink color change.
- Ames Test: Evaluates the mutagenic potential of chemicals by measuring the reversion of histidine auxotrophic bacteria to histidine prototrophy, often used in toxicology and environmental studies.
- Dinitrosalicylic Acid (DNS) Assay: Measures reducing sugars, particularly used in the analysis of sugar content in food and beverages.
- Barium Sulfate Precipitation Assay: Quantifies sulfate ions by forming a white precipitate with barium ions.
- Iron Thiocyanate Assay: Determines the concentration of iron ions by forming a red-colored complex with thiocyanate ions.
- Nitrate Test: Detects nitrate ions in water samples by forming a reddish-brown azo dye.
- Sulfanilamide-Diazotization Assay: Measures the concentration of nitrite ions by forming a diazo dye, often used in water quality analysis.
- Malachite Green Assay: Quantifies phosphate ions in solution by forming a green-colored complex.
- Cresol Red Assay: Measures the concentration of carbonate ions by changing the color of the solution from yellow to red.
- Methyl Orange Alkalinity Test: Determines alkalinity in water samples by monitoring a color shift from red to yellow.
- Chlorophyll Assay: Quantifies chlorophyll content in plant samples by measuring the absorbance at specific wavelengths.
Experimental Procedures:
- Title and Introduction:
- Provide a clear and concise title that reflects the experiment’s purpose.
- Give a brief introduction explaining the background, context, and objectives of the experiment.
- Materials and Equipment:
- List all materials, chemicals, reagents, and equipment needed for the experiment.
- Include specifications, quantities, and sources if necessary.
- Safety Precautions:
- Outline safety measures and precautions to be taken during the experiment, including handling of hazardous materials, protective equipment, and emergency procedures.
- Experimental Design:
- Describe the overall experimental design, including the research hypothesis, variables (independent, dependent, and control), and the rationale behind the chosen approach.
- Sample Preparation:
- Detail how samples, specimens, or test materials are prepared or obtained before the experiment begins.
- Procedure Steps:
- Provide a step-by-step account of the experimental procedure. Each step should be clear, specific, and concise.
- Include precise measurements, volumes, concentrations, and any timing requirements.
- Data Collection:
- Specify how data will be collected, including the type of measurements, instruments used, and the frequency of data recording.
- Note any factors that could affect data quality, such as environmental conditions.
- Data Analysis:
- Explain how data will be analyzed, including any calculations, statistical methods, or software tools to be used.
- Define parameters, variables, and units used in data analysis.
- Control Experiments:
- Describe any control experiments or conditions that are essential for comparing results.
- Explain why these controls are necessary.
- Data Presentation:
- Outline how data will be presented, such as tables, graphs, or figures.
- Specify any labeling or formatting conventions.
- Results and Observations:
- Record any observations, unexpected results, or deviations from expected outcomes.
- Include both qualitative and quantitative observations.
- Discussion:
- Analyze and interpret the results in the context of the research objectives.
- Discuss the implications of the findings and any potential sources of error.
- Conclusion:
- Summarize the key findings and their significance.
- Address whether the research objectives were met and suggest future research directions if applicable.
- References:
- Cite any sources, references, or prior research that informed the experimental design or interpretation of results.
- Appendices:
- Include any supplementary materials, such as raw data, calculations, or additional details that support the experimental procedures.
- Acknowledgments:
- Acknowledge individuals, organizations, or funding sources that contributed to the research.
Data Analysis and Interpretation:
Data analysis and interpretation are critical steps in the research process, involving the examination and making sense of collected data to draw meaningful conclusions and insights. Depending on the type of data and research objectives, various techniques and methods can be employed. Here’s a general guide to data analysis and interpretation:
1. Data Cleaning and Preprocessing:
- Begin by reviewing the collected data for errors, missing values, outliers, and inconsistencies.
- Address any issues through data cleaning and preprocessing, which may involve data imputation, transformation, or standardization.
2. Descriptive Statistics:
- Calculate basic descriptive statistics to summarize and characterize the data, including measures such as mean, median, mode, variance, standard deviation, and range.
- Create summary tables and visualizations (e.g., histograms, box plots) to provide an overview of the data’s distribution.
3. Exploratory Data Analysis (EDA):
- Conduct EDA to explore relationships, patterns, and trends in the data.
- Use scatterplots, correlation matrices, and other visualizations to identify potential associations between variables.
4. Hypothesis Testing:
- If applicable, formulate hypotheses based on the research questions or objectives.
- Choose appropriate statistical tests (e.g., t-tests, chi-square tests, ANOVA) to test hypotheses and assess the significance of differences or relationships in the data.
5. Regression Analysis:
- Perform regression analysis to model relationships between variables, such as linear regression for continuous outcomes or logistic regression for binary outcomes.
- Evaluate the coefficients, p-values, and goodness-of-fit to assess the strength and significance of relationships.
6. Data Visualization:
- Create data visualizations, including graphs, charts, and plots, to illustrate key findings and patterns.
- Use appropriate visualization tools (e.g., bar charts, scatterplots, heatmaps) for different types of data.
7. Qualitative Data Analysis:
- If dealing with qualitative data (e.g., interview transcripts, open-ended survey responses), use methods like content analysis or thematic analysis to identify recurring themes and patterns.
8. Statistical Software:
- Utilize statistical software packages (e.g., R, Python with libraries like Pandas, Matplotlib, Seaborn) to conduct data analysis efficiently and accurately.
9. Interpretation:
- Interpret the results of statistical analyses in the context of the research objectives and hypotheses.
- Explain the practical implications and significance of the findings.
10. Discussion and Conclusion: – In the discussion section of a research paper or report, relate the data analysis results to the research questions and objectives. – Discuss the implications of the findings, their limitations, and potential areas for further research. – Formulate a clear and concise conclusion that summarizes the key findings.
11. Data Validation: – Validate the robustness of the findings through sensitivity analysis or cross-validation, especially if the analysis involves predictive models.
12. Peer Review: – If applicable, undergo peer review or external validation of the data analysis and interpretation to ensure the rigor and accuracy of the results.
Advantages and Limitations:
Advantages and limitations are important aspects to consider when using colorimetric assays or any analytical technique. They help researchers and analysts understand the strengths and weaknesses of a method, allowing them to make informed decisions about its applicability for a particular situation. Here are some advantages and limitations of colorimetric assays:
Advantages:
- Simplicity: Colorimetric assays are relatively easy to perform and often require minimal training. This makes them accessible to a wide range of users.
- Cost-Effective: Compared to some other analytical techniques, colorimetric assays are cost-effective, as they don’t typically require expensive equipment or reagents.
- Speed: Colorimetric assays can provide results quickly, which is crucial in situations where rapid analysis is required, such as in clinical diagnostics.
- Quantitative Analysis: Colorimetric assays can provide quantitative data, allowing for precise measurement of analyte concentrations.
- Versatility: They can be applied to a wide range of analytes, including ions, small molecules, proteins, nucleic acids, and more, making them valuable in various fields.
- Automation: Many colorimetric assays can be automated, increasing throughput and reducing the potential for human error.
- High Sensitivity: Some colorimetric assays can achieve high levels of sensitivity, enabling the detection of trace amounts of analytes.
Limitations:
- Selectivity: Colorimetric assays may lack selectivity for specific analytes when multiple substances in the sample can produce a similar color change. This can lead to false-positive or false-negative results.
- Interference: Interfering substances in the sample can affect the accuracy of colorimetric assays. It’s essential to account for potential interference when designing experiments.
- Limited Dynamic Range: Colorimetric assays may have a limited dynamic range, meaning they can accurately measure analyte concentrations only within a certain range. Dilution or concentration steps may be needed for samples outside this range.
- Sample Complexity: Complex sample matrices, such as those found in biological fluids or environmental samples, can complicate colorimetric analysis and require additional sample preparation steps.
- Specificity: Some colorimetric assays may lack specificity when analytes share similar chemical properties or structures, leading to cross-reactivity.
- Instrumentation: While many colorimetric assays can be performed with basic equipment, higher precision and accuracy may require more advanced spectrophotometers, which can be expensive.
- Limited Information: Colorimetric assays provide information about the concentration of an analyte but may not offer insights into the compound’s identity or structure.
- Lack of Real-Time Monitoring: Colorimetric assays are typically endpoint assays, meaning they provide a measurement at a specific time point. Real-time monitoring of dynamic processes is not their strength.
Future Directions in Colorimetric Assays:
The field of colorimetric assays continues to evolve with advancements in technology, materials science, and analytical methods. Future directions in colorimetric assays are likely to focus on addressing current limitations, enhancing sensitivity and specificity, and expanding their applications. Here are some potential future directions in colorimetric assays:
- Nanomaterials and Nanotechnology: Integration of nanomaterials, such as nanoparticles and nanocomposites, can enhance the sensitivity and specificity of colorimetric assays. These materials can provide a high surface area for reactions and improve the detection of low-concentration analytes.
- Point-of-Care Diagnostics: Colorimetric assays are well-suited for point-of-care diagnostic applications due to their simplicity and speed. Future developments may involve the creation of portable and user-friendly devices that can deliver rapid results for various diseases and conditions.
- Multiplexed Assays: Researchers are working on multiplexed colorimetric assays that can simultaneously detect multiple analytes in a single sample. This can be particularly valuable in clinical diagnostics and environmental monitoring.
- Smartphone-Based Detection: Utilizing smartphone cameras for colorimetric measurements can democratize access to diagnostic tools, enabling remote or resource-limited areas to perform assays with minimal equipment.
- Biological and Molecular Markers: Advancements in colorimetric assays for detecting specific biological and molecular markers, such as DNA, RNA, and proteins, can have a significant impact on research, clinical diagnostics, and personalized medicine.
- Environmental Monitoring: Colorimetric assays are being employed for the rapid detection of environmental pollutants, including heavy metals, toxins, and microbial contaminants, contributing to real-time monitoring of environmental quality.
- Integration with Microfluidics: Combining colorimetric assays with microfluidic technology can enable efficient and automated sample handling, reducing the required sample volume and enhancing precision.
- Quantum Dots and Plasmonic Nanoparticles: These advanced materials can be incorporated into colorimetric assays to improve sensitivity and enable the detection of analytes at extremely low concentrations.
- Bioinformatics and Data Analysis: Future developments may involve integrating colorimetric assay data with bioinformatics and machine learning techniques to improve data interpretation, reduce false positives, and enhance assay reliability.
- Customized Assay Development: Tailoring colorimetric assays to specific analytes and applications will continue to be a focus, allowing researchers to design assays with optimized performance characteristics.
- Environmental Sustainability: Efforts to make colorimetric assays more environmentally friendly by reducing the use of hazardous chemicals and minimizing waste generation will likely gain prominence.
- Education and Training: As colorimetric assays become more advanced and diverse, education and training programs will be essential to ensure that users can properly perform and interpret the assays.
- Interdisciplinary Collaborations: Collaborations between chemists, biologists, engineers, and clinicians will drive innovation in colorimetric assay development, fostering cross-disciplinary approaches to problem-solving.
FAQs:
1. What is a colorimetric assay?
- A colorimetric assay is an analytical technique that measures the concentration of a substance in a sample by detecting and quantifying changes in color produced during a specific chemical reaction.
2. How does a colorimetric assay work?
- Colorimetric assays work by taking advantage of the fact that certain chemical reactions produce colored products. The intensity of the color change is proportional to the concentration of the analyte being measured.
3. What are the applications of colorimetric assays?
- Colorimetric assays have a wide range of applications, including clinical diagnostics, environmental monitoring, food testing, drug discovery, and biochemical research.
4. What are some common examples of colorimetric assays?
- Common examples include the Bradford assay for protein quantification, ELISA for detecting antibodies and antigens, and various assays for measuring the concentration of specific ions, molecules, or analytes.
5. What is the Beer-Lambert law, and how is it related to colorimetric assays?
- The Beer-Lambert law describes the relationship between the concentration of an analyte in a solution, the path length of the sample, and the absorbance of light at a specific wavelength. Colorimetric assays use this law to quantify analyte concentrations based on absorbance measurements.
6. What are the advantages of using colorimetric assays?
- Advantages include simplicity, cost-effectiveness, speed, versatility, and the ability to provide quantitative data quickly.
7. What limitations are associated with colorimetric assays?
- Limitations include potential interference from other substances in the sample, limited dynamic range, and the requirement for specific reactions to produce color changes.
8. Are colorimetric assays suitable for detecting trace amounts of analytes?
- Yes, some colorimetric assays can be sensitive enough to detect trace amounts of analytes, especially when combined with advanced techniques and materials.
9. How can one select the appropriate colorimetric assay for a specific application?
- The choice of assay depends on the nature of the analyte, its concentration range, and the desired level of sensitivity. Consideration of potential interference and the need for specificity is also important.
10. Are colorimetric assays suitable for fieldwork or point-of-care testing?
- Yes, colorimetric assays are often used for point-of-care diagnostics and fieldwork due to their simplicity and rapid results.
Conclusion:
In conclusion, colorimetric assays represent a powerful and versatile tool in the realm of analytical chemistry and scientific research. Their simplicity, cost-effectiveness, and ability to provide quantitative data swiftly make them invaluable for a wide range of applications, from clinical diagnostics to environmental monitoring and beyond. As technology advances, we can anticipate further refinements and innovations in colorimetric assays, continuing to contribute significantly to our understanding of the natural world and improving our ability to address complex analytical challenges.
Possible References Used