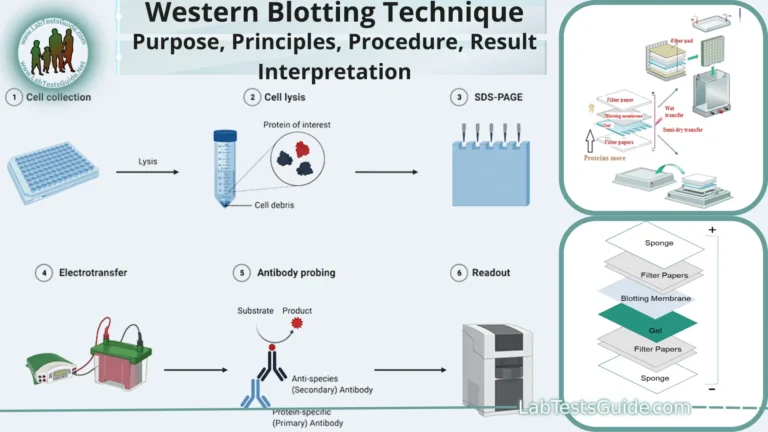
Blotting is a laboratory technique used in molecular biology and biochemistry to transfer biological molecules, such as DNA, RNA, or proteins, from a gel onto a solid support, typically a membrane. Blotting is crucial for various applications, including DNA and RNA hybridization, protein analysis, and nucleic acid sequencing.

Blotting is a technique used in molecular biology to transfer macromolecules, such as DNA, RNA, and proteins, from a gel to a solid surface for detection.
Definition and Purpose of Blotting:
Definition of Blotting:
Blotting is a laboratory technique used in molecular biology and biochemistry to transfer specific biological molecules, such as DNA, RNA, or proteins, from a gel matrix onto a solid support, typically a membrane. This technique allows for the subsequent detection, visualization, and analysis of these molecules, facilitating a wide range of research and diagnostic applications.
Purpose of Blotting:
The primary purposes of blotting techniques are as follows:
- Transfer and Detection: Blotting is used to transfer biological molecules separated within a gel (e.g., agarose or polyacrylamide gel) to a membrane surface. This transfer enables the molecules to be immobilized on the membrane, making them accessible for further analysis.
- Specific Detection: Blotting allows researchers to selectively detect and visualize specific molecules of interest within a complex mixture. For example, Southern blotting can be used to detect particular DNA sequences, Northern blotting for RNA sequences, and Western blotting for proteins.
- Analysis of Gene Expression: Blotting techniques are crucial for studying gene expression patterns. Northern blotting, for instance, is used to analyze the presence and quantity of specific mRNAs, providing insights into gene transcription and regulation.
- Protein Identification: Western blotting is widely employed to identify and quantify specific proteins in biological samples. It is valuable in various fields, including immunology and cancer research.
- Clinical Diagnostics: Blotting techniques are used in clinical laboratories for diagnosing genetic disorders, infectious diseases, and certain cancers. For example, the detection of viral DNA or RNA by blotting can confirm the presence of pathogens.
- Research Applications: Blotting is a fundamental tool in molecular biology and biochemistry research. It aids in investigating genetic variation, studying protein functions, and elucidating molecular mechanisms underlying diseases.
- Quality Control: In industries such as biotechnology and pharmaceuticals, blotting is used for quality control purposes to ensure the purity and identity of biological products.
- Forensic Analysis: DNA blotting, particularly Southern blotting, has been employed in forensic science to analyze DNA evidence and establish links between individuals and crime scenes.
Historical Overview:
A historical overview of blotting techniques provides insights into the development and evolution of these important laboratory methods in molecular biology and biochemistry:
1960s: The Emergence of Electrophoresis
- The foundation for blotting techniques was laid with the development of gel electrophoresis in the 1950s and 1960s. Gel electrophoresis allowed the separation of biological molecules, such as DNA, RNA, and proteins, based on their size and charge.
1970s: The Invention of Southern Blotting
- In 1975, Dr. Edwin Southern, a British biologist, introduced the Southern blotting technique. This revolutionary method allowed for the transfer of DNA fragments from an agarose gel onto a nitrocellulose membrane. The DNA on the membrane could then be probed with radioactive or fluorescent DNA fragments, enabling the detection of specific DNA sequences. Southern blotting became a cornerstone of DNA analysis, including gene mapping and DNA fingerprinting.
1980s: The Development of Northern Blotting and Western Blotting
- Following the success of Southern blotting, other blotting techniques were developed:
- In 1977, Joseph Sambrook and Peter Sharp introduced Northern blotting, which transferred RNA molecules from a gel to a membrane. This technique allowed researchers to study gene expression at the RNA level.
- In 1979, the Western blotting technique, initially known as protein blotting, was developed by Harry Towbin and colleagues. It involved transferring proteins from SDS-PAGE gels to a membrane and subsequently detecting specific proteins using antibodies. This technique revolutionized protein analysis and became an essential tool in immunology and cell biology.
1990s and Beyond: Advancements in Blotting Techniques
- The 1990s and subsequent decades saw significant advancements in blotting techniques, including:
- Improvements in Membrane Materials: Membranes made of nitrocellulose and nylon were widely used, and polyvinylidene difluoride (PVDF) membranes became popular due to their durability and versatility.
- Detection Methods: Enhanced detection methods, such as chemiluminescence and fluorescent labels, replaced radioactive probes for increased safety and sensitivity.
- Automation and High-Throughput Techniques: Automation systems were developed to streamline the blotting process, allowing for high-throughput analysis of samples.
- Integration with Genomic and Proteomic Research: Blotting techniques played a critical role in the Human Genome Project and continue to be integrated into large-scale genomics and proteomics studies.
Types of Blotting Techniques:
There are several types of blotting techniques used in molecular biology and biochemistry to transfer specific biological molecules from a gel onto a solid support (typically a membrane) for subsequent detection and analysis. Here are the main types of blotting techniques:
- Southern Blotting:
- Purpose: Southern blotting is used to transfer DNA fragments from an agarose gel to a solid support (e.g., nitrocellulose or nylon membrane) and then detect specific DNA sequences.
- Applications: It is commonly employed in genetic research for DNA fingerprinting, gene mapping, identifying DNA mutations, and studying gene expression by analyzing DNA fragments.
- Northern Blotting:
- Purpose: Northern blotting is designed to transfer RNA molecules (usually mRNA) from a gel to a membrane, allowing for the detection and quantification of specific RNA sequences.
- Applications: It is used to study gene expression by analyzing mRNA levels, identifying RNA splicing variants, and investigating regulatory processes at the transcriptional level.
- Western Blotting (Immunoblotting):
- Purpose: Western blotting transfers proteins separated by SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) to a membrane, enabling the detection of specific proteins using antibodies.
- Applications: Western blotting is widely used in immunology, cell biology, and biochemistry to identify and quantify target proteins, study protein post-translational modifications, and assess protein expression levels.
- Eastern Blotting:
- Purpose: Eastern blotting is a less common technique that transfers glycoconjugates (e.g., glycoproteins) separated by gel electrophoresis to a membrane for analysis.
- Applications: It is used to study glycosylation patterns of proteins and other molecules, which can have important implications for their functions.
- Southwestern Blotting:
- Purpose: Southwestern blotting combines DNA-binding and protein-detection techniques. It involves transferring proteins from a gel to a membrane and then probing the membrane with a labeled DNA probe to detect DNA-binding proteins (transcription factors).
- Applications: Southwestern blotting is used to study DNA-protein interactions and identify specific DNA-binding proteins in a sample.
- Far-Western Blotting:
- Purpose: Far-Western blotting is similar to Southwestern blotting but involves transferring denatured and renatured proteins to a membrane and then probing with labeled proteins or peptides to detect protein-protein interactions.
- Applications: It is used to study protein-protein interactions and identify specific protein partners or binding domains.
- Reverse Blotting:
- Purpose: Reverse blotting is a technique in which a sample is immobilized on a membrane, and complementary molecules (e.g., antibodies or DNA probes) are then applied to detect specific targets within the immobilized sample.
- Applications: Reverse blotting is used for various applications, such as protein arrays and antibody screening.
Other Blotting Techniques:
In addition to the well-known blotting techniques I mentioned earlier, there are several specialized blotting techniques that have been developed for specific applications. Here are some other blotting techniques:
- Northwestern Blotting:
- Purpose: Northwestern blotting is used to study RNA-binding proteins. It involves transferring denatured and renatured RNA molecules from a gel to a membrane, followed by probing with labeled RNA or DNA probes to detect RNA-binding proteins.
- Applications: Northwestern blotting helps identify and characterize RNA-binding proteins involved in post-transcriptional regulation.
- Zymography:
- Purpose: Zymography is a technique used to detect and quantify the activity of enzymes, such as proteases and glycosidases, in biological samples. It involves incorporating enzyme substrates into the gel, running electrophoresis, and then renaturing and staining to visualize enzyme activity.
- Applications: Zymography is commonly used in the study of enzyme secretion, tissue remodeling, and the identification of proteases in biological samples.
- Slot Blotting:
- Purpose: Slot blotting is a simplified version of Southern, Northern, or Western blotting that does not involve electrophoresis. Instead, samples are directly applied to a membrane through slots in a template, making it a quicker method for analyzing multiple samples.
- Applications: Slot blotting is useful for semi-quantitative analysis of DNA, RNA, or protein samples when traditional gel electrophoresis is not necessary.
- Dot Blotting:
- Purpose: Dot blotting is a highly simplified version of blotting in which samples are applied as small dots directly onto a membrane. It is often used for rapid screening or qualitative analysis of target molecules.
- Applications: Dot blotting can be used for various applications, such as antibody screening, antigen detection, and nucleic acid hybridization.
- Proteomic Blotting:
- Purpose: Proteomic blotting encompasses a range of techniques used for studying proteins, protein-protein interactions, and post-translational modifications. This includes techniques like Far-Western blotting, yeast two-hybrid blotting, and protein microarrays.
- Applications: Proteomic blotting methods are valuable for understanding protein functions, interactions, and modifications on a large scale, often in the context of systems biology.
- RNA Mobility Shift Assay (RNA EMSA):
- Purpose: RNA EMSA is used to study RNA-protein interactions. It involves incubating RNA molecules with proteins, separating them on a gel, transferring to a membrane, and then detecting the RNA-protein complexes.
- Applications: RNA EMSA is essential for investigating RNA-binding proteins, RNA secondary structure, and RNA-protein interactions involved in RNA processing and regulation.
Applications of Blotting Techniques:
Blotting techniques are widely used in molecular biology and biochemistry for various applications. These techniques are essential for transferring, detecting, and analyzing specific biological molecules, such as DNA, RNA, and proteins, in research, diagnostics, and other scientific endeavors. Here are some of the key applications of blotting techniques:
- Gene Mapping and DNA Fingerprinting (Southern Blotting):
- Southern blotting is employed to identify and locate specific DNA sequences within an individual’s genome.
- It is crucial in gene mapping, identifying genetic variations, and establishing DNA profiles for forensic purposes.
- Studying Gene Expression (Northern Blotting):
- Northern blotting is used to analyze gene expression patterns by quantifying mRNA levels.
- Researchers can study how genes are regulated and how their expression changes under different conditions or in various tissues.
- Protein Detection and Quantification (Western Blotting):
- Western blotting is a fundamental technique for detecting and quantifying specific proteins in complex mixtures.
- It is widely used in immunology, cell biology, and cancer research to study protein expression, post-translational modifications, and signaling pathways.
- Diagnosis of Genetic Disorders (Molecular Diagnostics):
- Blotting techniques are employed in clinical laboratories for diagnosing genetic disorders, including hereditary diseases and genetic mutations.
- They can confirm the presence or absence of specific genetic markers associated with diseases.
- Viral and Bacterial Infection Diagnosis (Nucleic Acid Detection):
- Blotting techniques are used to detect viral or bacterial DNA or RNA in patient samples, aiding in the diagnosis of infectious diseases.
- Examples include HIV detection through Western blotting and Southern blotting for hepatitis C virus.
- Research in Epigenetics (Methylation-Specific Blotting):
- Blotting techniques can be adapted for studying epigenetic modifications, such as DNA methylation.
- Methylation-specific blotting allows researchers to investigate changes in DNA methylation patterns associated with gene regulation and diseases like cancer.
- Protein-Protein and RNA-Protein Interaction Studies:
- Techniques like Southwestern blotting and RNA EMSA are used to investigate interactions between proteins and DNA/RNA.
- They help identify transcription factors, RNA-binding proteins, and their binding sites.
- Enzyme Activity Assays (Zymography):
- Zymography uses blotting to detect and quantify the activity of enzymes, including proteases and glycosidases.
- It is applied in studies related to tissue remodeling and enzyme secretion.
- Proteomic Analysis (Proteomics Blotting):
- Proteomic blotting methods, including Far-Western blotting and protein microarrays, facilitate the study of protein-protein interactions and post-translational modifications on a large scale.
- These techniques contribute to systems biology and biomarker discovery.
- Quality Control in Biotechnology and Pharmaceutical Industries:
- Blotting techniques are used for quality control purposes to ensure the purity and identity of biological products, including recombinant proteins and nucleic acids.
- Research in Structural Biology and Functional Genomics:
- Blotting is essential for characterizing the structure and function of biomolecules, aiding in the understanding of biological processes and disease mechanisms.
- Drug Discovery and Development:
- Blotting techniques are used in drug discovery programs to validate drug targets, assess the effects of potential therapeutics on specific proteins, and study signaling pathways.
Common Materials and Reagents:
In blotting techniques, several common materials and reagents are used to perform the experiments effectively. Here is a list of some of the commonly used materials and reagents in blotting techniques:
Materials:
- Gel Electrophoresis Setup:
- Agarose or polyacrylamide gels, depending on the type of molecules being separated (DNA, RNA, or proteins).
- Electrophoresis chamber with electrodes.
- Gel comb and casting tray.
- Transfer Setup:
- Nitrocellulose or nylon membrane: Solid support for transferring molecules from the gel.
- Transfer apparatus (e.g., tank or semi-dry blotting system).
- Filter paper or blotting pads.
- Membrane Handling Tools:
- Forceps or plastic spatula for handling membranes.
- Weighing paper or plastic wrap to protect the membrane during handling.
- Blocking and Washing Materials:
- Blocking reagents (e.g., non-fat dry milk, BSA, or casein) to block nonspecific binding sites on the membrane.
- Washing buffer (e.g., Tris-buffered saline with Tween 20, TBST) to rinse the membrane and remove unbound antibodies or probes.
- Incubation and Detection Tools:
- Microcentrifuge tubes or containers for incubation of membranes with antibodies or probes.
- Antibodies or labeled probes specific to the target molecule.
- Chemiluminescent or fluorescent substrates for detection (e.g., ECL substrate).
- Safety Equipment:
- Lab coats, gloves, and safety goggles to ensure proper safety precautions are followed when handling chemicals and biohazardous materials.
Reagents:
- Electrophoresis Buffer:
- TAE (Tris-Acetate-EDTA) or TBE (Tris-Borate-EDTA) buffer for DNA and RNA electrophoresis.
- SDS-PAGE running buffer for protein electrophoresis.
- Transfer Buffer:
- A transfer buffer specific to the type of blotting being performed (e.g., SSC buffer for Southern blotting, transfer buffer for Western blotting).
- Denaturation and Renaturation Reagents (if applicable):
- Denaturing solution (e.g., formamide) for denaturation of nucleic acids in RNA blots.
- Renaturation solution for RNA blots to restore secondary structure.
- Blocking Buffer:
- Blocking buffer containing a blocking agent (e.g., non-fat dry milk, BSA) to block nonspecific binding sites on the membrane.
- Primary and Secondary Antibodies:
- Primary antibodies specific to the target molecule (e.g., anti-protein antibodies, anti-DNA or RNA probes).
- Secondary antibodies conjugated to enzymes or fluorophores for signal detection (e.g., anti-rabbit IgG-HRP or anti-mouse IgG-Alexa Fluor 488).
- Chemiluminescent or Fluorescent Substrates:
- Chemiluminescent substrates for horseradish peroxidase (HRP) detection (e.g., luminol-based substrates).
- Fluorescent substrates for fluorescence-based detection (e.g., ECL substrates for fluorescence detection).
- Stains and Dyes:
- Ethidium bromide or SYBR Green for nucleic acid staining in DNA and RNA gels.
- Coomassie Blue or silver staining reagents for protein gels.
- Ethanol and Methanol:
- Used for dilution, preparation of transfer buffer, or other applications.
- Molecular Weight Markers:
- DNA, RNA, or protein markers for size determination and verification of the blotting process.
- Deionized Water:
- Used for diluting reagents and preparing buffers.
Experimental Design and Sample Preparation:
Experimental design and sample preparation are critical steps in any blotting technique. Proper planning and execution of these stages are essential to obtain meaningful and reproducible results. Below are the key considerations for experimental design and sample preparation in blotting techniques:
Experimental Design:
- Define the Research Question: Clearly articulate the research objectives and the specific molecules (DNA, RNA, proteins) you intend to study using the blotting technique.
- Choose the Appropriate Blotting Technique: Select the blotting method that best suits your research goals based on the type of molecule you are analyzing (e.g., Southern blotting for DNA, Northern blotting for RNA, Western blotting for proteins).
- Sample Size and Replicates: Determine the number of samples and replicates needed for statistical significance. Ensure that sample size is appropriate for the desired level of confidence in the results.
- Positive and Negative Controls: Include positive controls (samples with known target molecules) and negative controls (samples without the target molecules) to validate the specificity of the detection method.
- Experimental Conditions: Define the experimental conditions, such as temperature, incubation time, and pH, to be consistent throughout the experiment.
- Choice of Detection Method: Decide on the detection method, whether it’s chemiluminescence, fluorescence, or another technique, and select appropriate reagents and equipment accordingly.
Sample Preparation:
- Sample Collection and Handling:
- Collect and handle samples with care to prevent contamination and degradation.
- Ensure proper storage conditions (e.g., freezing at -80°C or using RNase/protease inhibitors for RNA or protein samples).
- Sample Homogenization and Lysis:
- Homogenize tissues or cells thoroughly to ensure uniform distribution of molecules.
- Use appropriate lysis buffers to extract DNA, RNA, or proteins.
- Sample Concentration and Purity:
- Assess the concentration and purity of nucleic acid samples using spectrophotometry (e.g., UV absorbance ratios) or other methods.
- For protein samples, determine protein concentration using assays like Bradford or BCA.
- DNA and RNA Denaturation (if applicable):
- Denature DNA or RNA samples by heating if necessary, typically for Southern and Northern blotting.
- Gel Electrophoresis:
- Prepare gels with the appropriate percentage and running conditions (e.g., voltage, run time) based on the size of the molecules to be separated.
- Load samples carefully, including molecular weight markers for size reference.
- Blotting Transfer:
- Transfer molecules from the gel to the membrane using the selected blotting method (e.g., electroblotting for proteins).
- Ensure even transfer of molecules to the membrane.
- Blocking and Incubation:
- Block the membrane to prevent nonspecific binding by incubating it in blocking buffer.
- Incubate the membrane with primary antibodies or probes specific to the target molecules.
- Washing Steps:
- Perform thorough washing steps with appropriate buffers to remove unbound antibodies or probes and reduce background noise.
- Secondary Antibodies (if applicable):
- If using Western blotting or similar techniques, apply secondary antibodies conjugated to enzymes or fluorophores.
- Signal Detection:
- Use the chosen detection method to visualize and quantify the target molecules (e.g., chemiluminescence, fluorescence, or colorimetry).
- Data Analysis:
- Analyze and interpret the results using appropriate software or imaging systems.
- Quantify the signal intensity and compare it to controls and standards.
- Replication and Validation:
- Repeat the experiment with replicates to ensure the reliability of the results.
- Verify the findings through additional experiments or complementary techniques.
- Documentation and Reporting:
- Keep detailed records of experimental procedures, including protocols, reagents, and equipment used.
- Prepare clear and concise reports or publications to communicate the results effectively.
Blotting Procedure:
The blotting procedure can vary depending on the specific blotting technique you are using (Southern, Northern, Western, etc.) and the type of biological molecules (DNA, RNA, or proteins) you want to analyze. However, here’s a general overview of the steps involved in blotting procedures:
1. Sample Preparation:
- Start by preparing your samples, which may involve isolating and purifying DNA, RNA, or proteins from your biological material.
- Assess the quality and quantity of your samples using spectrophotometry or other relevant assays.
2. Gel Electrophoresis:
- For DNA and RNA analysis (Southern or Northern blotting), run an agarose or polyacrylamide gel, respectively, to separate the molecules based on size.
- For protein analysis (Western blotting), perform SDS-PAGE to separate proteins by molecular weight.
3. Transfer to Membrane:
- Prepare a transfer apparatus appropriate for the blotting technique you’re using (e.g., electroblotting for proteins).
- Transfer the separated molecules from the gel to a solid support membrane (typically nitrocellulose or nylon) using transfer buffer and appropriate voltage or current.
4. Blocking:
- Block the membrane with a blocking buffer (e.g., non-fat dry milk, BSA) to prevent nonspecific binding of antibodies or probes.
- Incubate the membrane in the blocking buffer for an appropriate amount of time.
5. Incubation with Primary Antibodies or Probes:
- Incubate the membrane with primary antibodies specific to your target molecules (e.g., anti-DNA, anti-RNA, or anti-protein antibodies) or labeled DNA/RNA probes.
- Allow the primary antibodies or probes to bind to their respective targets on the membrane.
6. Washing:
- Wash the membrane multiple times with a suitable washing buffer (e.g., TBST for Western blotting) to remove unbound antibodies or probes.
7. Secondary Antibodies (if applicable):
- If using Western blotting or similar techniques, incubate the membrane with secondary antibodies conjugated to enzymes (e.g., HRP) or fluorophores.
- Secondary antibodies bind to the primary antibodies and enhance signal detection.
8. Washing (Secondary Antibodies):
- Wash the membrane again to remove unbound secondary antibodies.
9. Signal Detection:
- Detect the target molecules by using an appropriate detection method:
- For Western blotting: Use chemiluminescence, fluorescence, or colorimetry to visualize protein bands.
- For Southern and Northern blotting: Use autoradiography, chemiluminescence, or fluorescence to detect DNA or RNA bands.
10. Image Capture:
- Capture images of the membrane with the detected signal using a chemiluminescent or fluorescent imager or X-ray film (for autoradiography).
11. Data Analysis:
- Analyze and quantify the signals, comparing them to molecular weight markers, controls, and standards.
- Use appropriate software or imaging systems for data analysis.
12. Documentation and Reporting:
- Document all steps of the procedure, including protocols, reagents, and results.
- Prepare clear and concise reports or publications to communicate your findings effectively.
Data Analysis and Interpretation:
Data analysis and interpretation are crucial steps in blotting techniques, as they enable you to derive meaningful information from the obtained results. The specific methods and tools for data analysis can vary depending on the type of blotting (Southern, Northern, Western, etc.) and the type of molecules (DNA, RNA, or proteins) you are studying. Here’s a general overview of data analysis and interpretation in blotting techniques:
1. Image Acquisition:
- Capture clear and high-quality images of the blots using appropriate imaging equipment (e.g., chemiluminescence or fluorescence imaging systems, X-ray film, or a gel documentation system).
2. Background Subtraction:
- If necessary, subtract background noise from the images to enhance the clarity of bands or signals.
3. Quantification:
- Quantify the intensity of bands or signals corresponding to your target molecules using suitable software or analysis tools.
- For densitometry (quantification of signal intensity), select regions of interest (ROIs) around the bands and measure pixel intensities.
4. Standard Curves and Controls:
- If applicable, use standard curves generated from known concentrations or quantities of standards or controls to convert signal intensity into absolute values (e.g., concentration or expression level).
5. Normalization:
- Normalize the data to account for variations in sample loading and transfer efficiency. Common normalization methods include:
- Using housekeeping genes or internal controls for RNA or protein blots.
- Staining gels with DNA, RNA, or protein dyes to visualize total loaded material for nucleic acid or protein blots.
6. Statistical Analysis:
- Perform statistical analysis, such as t-tests, ANOVA, or non-parametric tests, to assess the significance of differences between experimental groups.
- Calculate standard deviations, confidence intervals, and p-values as appropriate.
7. Data Presentation:
- Present your data in a clear and informative manner using graphs, tables, or figures.
- Include error bars to visualize data variability, if applicable.
8. Interpretation:
- Interpret the results based on the objectives of your study and the patterns observed in the blots.
- Look for specific bands or signals corresponding to your target molecules.
- Compare the intensity and position of bands between samples, controls, and standards.
9. Quality Control:
- Check for anomalies or inconsistencies in your data. Ensure that the bands correspond to the expected molecular weights or sizes.
- Verify that controls and standards behave as expected.
10. Biological Implications:
- Relate your findings to the biological context of your study. What do the results suggest about the presence, abundance, or regulation of your target molecules?
- Consider the potential biological significance of your findings.
11. Reproducibility:
- Ensure that your results are reproducible by repeating the experiment with replicates.
- Verify that similar results are obtained across multiple experimental runs.
12. Discussion and Conclusion:
- Discuss the implications of your results in the broader context of your research question.
- Draw conclusions based on your data and offer insights or hypotheses for further investigation.
Advancements and Variations:
Advancements and variations in blotting techniques have played a crucial role in expanding the capabilities and applications of these methods. Over the years, researchers have developed innovative approaches and modifications to improve sensitivity, accuracy, and versatility in studying DNA, RNA, and proteins. Here are some notable advancements and variations in blotting techniques:
Advancements:
- Enhanced Detection Methods:
- Chemiluminescent and fluorescent detection systems have largely replaced radioactive labeling for safer and more sensitive detection of target molecules.
- High-Throughput Blotting:
- Automation and robotics have been integrated into blotting workflows, allowing for high-throughput analysis of samples, particularly in genomics and proteomics research.
- Multiplexing:
- Multiplex blotting techniques enable the simultaneous detection of multiple targets within a single sample, saving time and resources. For example, protein microarrays and bead-based assays.
- Digital Imaging and Quantification:
- Advanced imaging systems and software allow for precise quantification of signals, improving accuracy and reproducibility in data analysis.
- Chemifluorescence:
- Chemifluorescent substrates combine the advantages of chemiluminescence and fluorescence, offering excellent sensitivity and compatibility with fluorescent detection systems.
- Nanoblotting:
- Nanoblotting techniques have been developed to detect and analyze biological molecules at the nanoscale level, allowing for the study of individual molecules or single cells.
- Integration with Mass Spectrometry:
- Blotting techniques have been integrated with mass spectrometry for protein identification and post-translational modification analysis, expanding their applications in proteomics.
- Customized Probes and Antibodies:
- Advances in molecular biology techniques, such as PCR and recombinant DNA technology, have facilitated the development of highly specific probes and antibodies for blotting.
Variations:
- Southern Blotting Variations:
- Inverse Southern blotting detects restriction enzyme recognition sites in DNA.
- Genomic Southern blotting assesses DNA methylation patterns.
- Northern Blotting Variations:
- RNase protection assays provide high sensitivity for quantifying specific RNA transcripts.
- Ribonuclease mapping allows for the precise determination of RNA secondary structures.
- Western Blotting Variations:
- Phospho-specific antibodies detect phosphorylated proteins in phospho-Western blotting.
- Far-Western blotting investigates protein-protein interactions.
- Proximity ligation assay (PLA) combines Western blotting and immunoprecipitation to study protein-protein interactions.
- Proteomic Blotting Variations:
- Yeast two-hybrid blotting assesses protein-protein interactions in a high-throughput manner.
- Reverse-phase protein arrays (RPPAs) enable the analysis of protein expression and phosphorylation profiles in cancer research.
- In-Cell Western Assays:
- In-cell Western assays perform protein quantification directly in live cells, providing insights into cellular responses and signaling pathways.
- Rolling Circle Amplification Blotting:
- This technique amplifies circular DNA probes on the blotting membrane, enhancing sensitivity and signal detection.
- Single-Cell RNA Blotting:
- Single-cell RNA blotting techniques allow for the analysis of RNA transcripts at the single-cell level, revealing heterogeneity in gene expression within a population.
Comparative Analysis of Blotting Techniques:
Comparative analysis of blotting techniques involves evaluating the advantages, limitations, and applications of various blotting methods (Southern, Northern, Western, etc.) to determine which one is most suitable for a specific research question or experimental goal. Here, we’ll provide a comparative analysis of some commonly used blotting techniques:
1. Southern Blotting:
- Molecules Detected: DNA fragments.
- Applications:
- Genetic mapping.
- DNA fingerprinting.
- Studying DNA methylation.
- Identifying DNA mutations.
- Advantages:
- High specificity for DNA sequences.
- Can analyze large DNA fragments.
- Limitations:
- Time-consuming and labor-intensive.
- Requires radioactive or non-radioactive probes.
- Sensitivity: Medium to high, depending on probe and detection method.
- Throughput: Low to moderate.
2. Northern Blotting:
- Molecules Detected: RNA molecules, typically mRNA.
- Applications:
- Gene expression analysis.
- Studying RNA splicing variants.
- Investigating post-transcriptional regulation.
- Advantages:
- Provides information about RNA size and abundance.
- High specificity for RNA sequences.
- Limitations:
- Time-consuming and labor-intensive.
- Requires radioactive or non-radioactive probes.
- Sensitivity: Medium to high, depending on probe and detection method.
- Throughput: Low to moderate.
3. Western Blotting (Immunoblotting):
- Molecules Detected: Proteins.
- Applications:
- Protein detection and quantification.
- Studying post-translational modifications.
- Assessing protein expression in cells and tissues.
- Advantages:
- High specificity for target proteins.
- Versatile for various protein sizes and types.
- Can analyze multiple proteins in a single sample.
- Limitations:
- Antibody quality and specificity are critical.
- Limited by the availability of specific antibodies.
- Some proteins may not transfer or bind well.
- Sensitivity: High, with chemiluminescent and fluorescent detection.
- Throughput: Moderate to high, especially with automation.
4. Eastern Blotting:
- Molecules Detected: Glycoconjugates, such as glycoproteins.
- Applications:
- Analyzing glycosylation patterns of proteins.
- Advantages:
- Provides insights into protein glycosylation.
- Limitations:
- Requires specific lectins or antibodies for detection.
- Sensitivity: Moderate.
- Throughput: Low to moderate.
5. Southwestern Blotting:
- Molecules Detected: DNA-binding proteins (transcription factors).
- Applications:
- Identifying and studying DNA-binding proteins.
- Advantages:
- Can detect protein-DNA interactions.
- Limitations:
- Requires labeled DNA probes and specific conditions.
- Sensitivity: Moderate.
- Throughput: Low to moderate.
6. Proteomic Blotting (e.g., Far-Western Blotting):
- Molecules Detected: Protein-protein interactions, post-translational modifications.
- Applications:
- Studying protein-protein interactions.
- Identifying specific protein partners.
- Advantages:
- Provides insights into protein function and interactions.
- Limitations:
- Requires specific probes or bait proteins.
- Sensitivity: Variable, depending on the method.
- Throughput: Low to moderate.
Ethical and Safety Considerations:
When conducting blotting experiments or any laboratory work in the field of molecular biology, it is essential to adhere to ethical and safety considerations to ensure the well-being of researchers, the responsible use of materials, and the integrity of research outcomes. Here are some key ethical and safety considerations for blotting experiments:
Ethical Considerations:
- Ethical Treatment of Research Subjects:
- If human or animal samples are involved, ensure that research involving these subjects complies with ethical guidelines and is approved by the appropriate ethics committees or institutional review boards (IRBs).
- Informed Consent:
- When working with human samples, obtain informed consent from participants, ensuring they understand the purpose and potential risks of the research.
- Data Privacy:
- Safeguard the privacy and confidentiality of individuals whose data or samples are used in research. Adhere to data protection regulations and secure data storage and sharing.
- Publication Ethics:
- Follow ethical guidelines for the publication and dissemination of research results. Avoid data manipulation or fraudulent practices.
- Conflict of Interest:
- Disclose any potential conflicts of interest that could bias research outcomes or the interpretation of results.
Safety Considerations:
- Laboratory Safety:
- Adhere to laboratory safety protocols, including the use of appropriate personal protective equipment (PPE) such as lab coats, gloves, and safety goggles.
- Chemical Safety:
- Handle chemicals, reagents, and staining agents with care, following Material Safety Data Sheet (MSDS) guidelines.
- Label and store chemicals properly.
- Radioactive Materials (if applicable):
- If working with radioactive probes or materials, ensure compliance with radiation safety regulations, including proper shielding, monitoring, and disposal.
- Biological Safety:
- Follow biosafety protocols when working with biological materials, including proper disposal of biohazardous waste.
- Electrical Safety:
- Ensure that electrical equipment, such as power supplies and gel documentation systems, is in good working order and properly grounded.
- Equipment Safety:
- Familiarize yourself with the safe operation of laboratory equipment, such as electrophoresis apparatus and blotting systems.
- Waste Disposal:
- Dispose of laboratory waste, including gels, membranes, and chemical reagents, in accordance with local regulations and guidelines.
- Emergency Procedures:
- Be aware of emergency procedures, including the location of safety showers, eyewash stations, and fire extinguishers.
- Training and Education:
- Ensure that all laboratory personnel are properly trained in the techniques and safety procedures relevant to their work.
- Risk Assessment:
- Conduct risk assessments for specific experiments to identify potential hazards and implement appropriate safety measures.
- Record Keeping:
- Maintain accurate records of experimental procedures, safety precautions, and any incidents or accidents that occur in the laboratory.
- Collaborative Research:
- If collaborating with other research institutions or laboratories, ensure that safety and ethical standards are consistent across all partners.
- Emergency Contacts:
- Have emergency contact information readily available in the laboratory, including contact information for safety officers and medical facilities.
Future Trends in Techniques:
The field of blotting techniques has seen significant advancements over the years, and it continues to evolve as researchers seek more sensitive, efficient, and versatile methods for analyzing DNA, RNA, and proteins. Here are some future trends and developments that may shape the landscape of blotting techniques:
- Digital and Miniaturized Blotting:
- Future blotting techniques may become more miniaturized and automated, allowing for high-throughput analysis of multiple samples simultaneously.
- Digital blotting systems may provide real-time data acquisition and analysis, reducing the time and labor required for experiments.
- Enhanced Sensitivity and Detection:
- Ongoing developments in detection technologies, including advancements in chemiluminescent, fluorescent, and electrochemical detection, will likely continue to improve sensitivity in blotting experiments.
- New labels and detection methods may enable the detection of even trace amounts of molecules.
- Single-Cell Blotting:
- Single-cell blotting techniques will likely gain prominence, enabling the analysis of DNA, RNA, and proteins at the individual cell level. This will provide insights into cellular heterogeneity and responses.
- Integration with High-Throughput Omics Technologies:
- Blotting techniques may be integrated with other high-throughput omics technologies, such as next-generation sequencing and mass spectrometry, to provide comprehensive molecular profiling.
- Multiplexed and Imaging-Based Blotting:
- Multiplexed blotting methods, allowing simultaneous detection of multiple targets in a single sample, will continue to evolve.
- Advanced imaging technologies may offer more detailed and quantitative information about the spatial distribution of molecules in tissues and cells.
- Customized and Personalized Blotting:
- Researchers may develop customized blotting methods tailored to specific research questions or clinical applications, enabling personalized diagnostics and treatments.
- Customized probes and antibodies could become more accessible and affordable.
- Label-Free Blotting:
- Label-free blotting techniques that eliminate the need for labeling probes or antibodies may gain popularity, reducing the complexity of experiments and potential sources of variability.
- High-Resolution Blotting:
- Advances in gel electrophoresis and blotting systems may lead to higher resolution in separating and transferring molecules, allowing for finer molecular analysis.
- Data Integration and Systems Biology:
- Blotting data may be integrated with other -omics data (genomics, transcriptomics, proteomics, metabolomics) to provide a more comprehensive understanding of biological systems and pathways.
- Point-of-Care and Clinical Applications:
- Blotting techniques may find increased use in point-of-care diagnostics, providing rapid and sensitive tests for diseases and biomarkers in clinical settings.
- Sustainable and Green Techniques:
- Researchers may develop more environmentally friendly blotting techniques that reduce the use of hazardous chemicals and minimize waste generation.
- AI and Machine Learning Integration:
- Artificial intelligence (AI) and machine learning algorithms may be employed for data analysis, pattern recognition, and predictive modeling in blotting experiments.
- Education and Accessibility:
- Efforts to make blotting techniques more accessible and user-friendly, including educational resources and open-source tools, may empower researchers from diverse backgrounds to utilize these methods.
FAQs:
1. What is the purpose of blotting techniques?
Blotting techniques are used to transfer, detect, and analyze specific biological molecules, such as DNA, RNA, and proteins, from a gel to a solid support membrane. They are commonly used in molecular biology and biochemistry research to study gene expression, identify specific molecules, and investigate molecular interactions.
2. What are the common types of blotting techniques?
The common types of blotting techniques include Southern blotting (for DNA), Northern blotting (for RNA), and Western blotting (for proteins). There are also variations and specialized techniques for specific applications.
3. How does Western blotting work?
Western blotting involves the separation of proteins by SDS-PAGE, transfer to a membrane, and subsequent detection using antibodies specific to the target proteins. It is commonly used for protein detection and quantification.
4. What is the difference between Northern and Southern blotting?
Northern blotting is used to study RNA molecules, particularly mRNA, while Southern blotting is used for DNA analysis. The key difference is the type of molecule being detected.
5. Are there alternatives to radioactive probes in blotting techniques?
Yes, non-radioactive alternatives, such as chemiluminescent and fluorescent probes, are commonly used for detection in blotting techniques. They are safer and offer similar or better sensitivity than radioactive probes.
6. What safety precautions should be taken when working with blotting techniques?
Safety precautions include wearing appropriate personal protective equipment (PPE), following laboratory safety protocols, and handling chemicals, radioactive materials (if applicable), and biohazardous materials with care.
7. How can I increase the sensitivity of my blotting experiments?
You can increase sensitivity by optimizing the choice of detection method, using high-quality antibodies or probes, and ensuring efficient transfer of molecules from the gel to the membrane.
8. What are some applications of blotting techniques in research?
Blotting techniques are used in a wide range of applications, including gene expression analysis, disease diagnostics, protein identification, and the study of molecular interactions.
9. Are there variations of blotting techniques for specialized purposes?
Yes, there are variations like reverse Northern blotting, Southwestern blotting, Far-Western blotting, and many others, each tailored to specific research needs.
10. How can I troubleshoot common issues in blotting experiments?
Common issues in blotting experiments include poor signal, high background, and inconsistent results. Troubleshooting involves optimizing experimental conditions, reagents, and detection methods, as well as verifying the quality of antibodies and probes.
Conclusion:
In conclusion, blotting techniques have been indispensable tools in molecular biology and biochemistry for several decades. They enable the transfer, detection, and analysis of DNA, RNA, and proteins, allowing researchers to explore gene expression, identify specific molecules, and investigate molecular interactions. Blotting methods, such as Southern, Northern, and Western blotting, have evolved significantly over time, with advancements in sensitivity, automation, and versatility.
Researchers continue to push the boundaries of blotting techniques, exploring new frontiers in single-cell analysis, label-free detection, high-throughput applications, and integration with other omics technologies. As the field advances, ethical and safety considerations remain paramount to ensure responsible and safe laboratory practices.
Blotting techniques are poised for a future characterized by increased sensitivity, automation, and integration with emerging technologies, making them powerful tools for understanding the complexities of DNA, RNA, and protein biology. With ongoing research and innovation, blotting methods will continue to contribute to our understanding of the molecular basis of life and impact diverse fields, from fundamental research to clinical diagnostics and personalized medicine.
Possible References Used