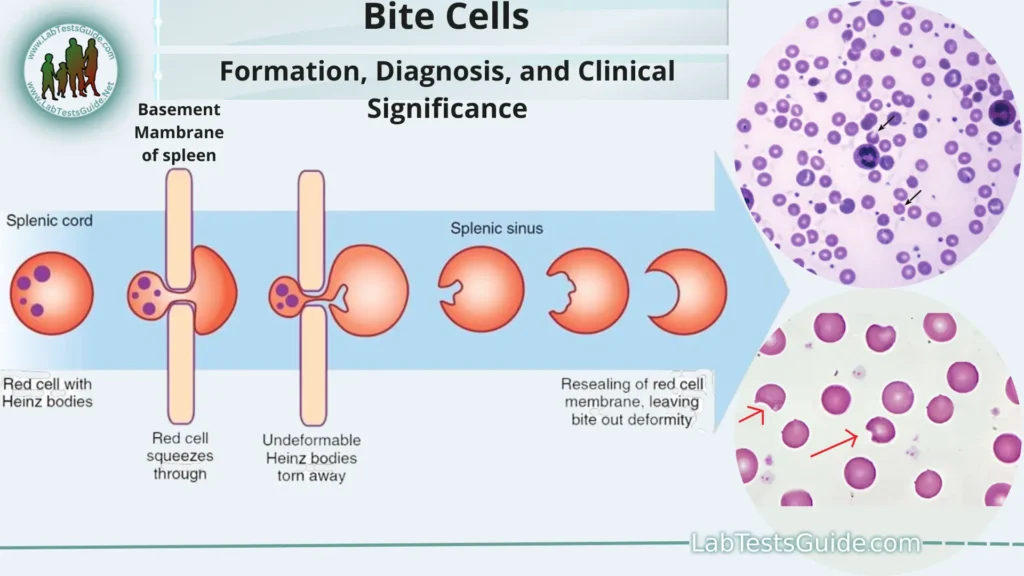
Abnormally shaped red blood cells with one or more semicircular portions removed from the cell margin, the cells appearing to have been “bitten” or “bitten”. “Stings” result from the removal of hemoglobin with an altered (denatured) structure by special cells (macrophages) in the spleen. G6PD deficiency is a common disorder that leads to the formation of bite cells, which can be seen in a stained blood smear.
What are Bite Cells?
Bite cells are a type of abnormal red blood cell that can be observed in a peripheral blood smear when examining a patient’s blood under a microscope. These cells are characterized by a distinctive appearance, which includes a semicircular or “bitten” shape along the edge of the red blood cell membrane. Bite cells are typically associated with a medical condition known as G6PD (glucose-6-phosphate dehydrogenase) deficiency, which is a genetic disorder that affects red blood cells.
Significance in Hematology:
Here are some key points regarding the significance of bite cells in hematology.
- Diagnostic Marker: Bite cells are an important diagnostic marker for G6PD deficiency. When healthcare professionals observe bite cells in a peripheral blood smear, it raises suspicion of G6PD deficiency and can prompt further testing for confirmation.
- Identification of Hemolysis: Bite cells are indicative of hemolysis, the premature destruction of red blood cells. This information is crucial in diagnosing and monitoring various hemolytic disorders, including those beyond G6PD deficiency.
- Differentiating Causes of Hemolysis: While bite cells are associated with G6PD deficiency, other features observed in a peripheral blood smear, such as spherocytes or schistocytes, can help differentiate the underlying cause of hemolysis. This aids in determining the appropriate treatment and management.
- Treatment Guidance: Identifying bite cells can guide healthcare providers in tailoring treatment and management strategies for individuals with G6PD deficiency. It helps in avoiding specific triggers and complications associated with the condition.
- Research and Clinical Studies: Bite cells are also of interest in hematology research and clinical studies. Understanding the mechanisms behind their formation and their role in various hemolytic conditions can lead to advancements in the diagnosis and treatment of these disorders.
- Patient Education: Recognizing the presence of bite cells in a blood smear can be part of patient education. It helps individuals with G6PD deficiency and their caregivers understand the importance of avoiding triggers that can exacerbate hemolysis and manage their condition effectively.
G6PD Deficiency:
Here is an overview of G6PD deficiency.
- Genetic Basis: G6PD deficiency is inherited in an X-linked recessive manner. This means that the gene responsible for G6PD is located on the X chromosome. Individuals with two X chromosomes (females) may be carriers or affected, while individuals with one X and one Y chromosome (males) are more likely to be affected if they inherit a defective G6PD gene.
- Role of G6PD Enzyme: The G6PD enzyme plays a crucial role in protecting red blood cells from oxidative damage. It is involved in the pentose phosphate pathway, which generates molecules necessary for the cell’s defense against oxidative stress.
Oxidative Stress and G6PD Deficiency:
- People with G6PD deficiency have lower levels of functional G6PD enzyme, making their red blood cells more vulnerable to oxidative stress. This can be triggered by various factors, including:
- Certain medications (e.g., sulfa drugs, antimalarials)
- Infections (e.g., viral or bacterial infections)
- Fava bean consumption
- Chemical exposure (e.g., naphthalene in mothballs)
Hemolysis and Anemia:
- When red blood cells in individuals with G6PD deficiency are exposed to oxidative stress, they become more fragile and prone to breaking apart (hemolysis). This leads to a reduction in the number of circulating red blood cells and can result in hemolytic anemia.
- Symptoms of hemolytic anemia may include fatigue, pale skin, jaundice (yellowing of the skin and eyes), dark urine, and abdominal pain.
- Variability in Symptoms: The severity of G6PD deficiency and the frequency of hemolytic episodes can vary widely among individuals. Some may experience mild, intermittent symptoms, while others may have severe and life-threatening episodes.
- Diagnosis: G6PD deficiency is typically diagnosed through blood tests that measure G6PD enzyme activity. It is essential to confirm the diagnosis because some individuals may have mild deficiencies that do not cause symptoms.
- Management and Treatment: Management of G6PD deficiency primarily involves avoiding triggers that can cause oxidative stress and hemolysis. This includes avoiding specific medications, foods (like fava beans), and situations that may lead to infection.
In severe cases of hemolysis, supportive care and blood transfusions may be necessary. - Prognosis: With proper management and avoidance of triggers, individuals with G6PD deficiency can lead normal, healthy lives. The prognosis varies depending on the severity of the deficiency and the diligence of trigger avoidance.
Formation of Bite Cells:
- G6PD Deficiency: The formation of bite cells is primarily associated with G6PD deficiency, a genetic disorder that affects the enzyme glucose-6-phosphate dehydrogenase. This enzyme is essential for protecting red blood cells from oxidative damage.
- Oxidative Stress: When individuals with G6PD deficiency are exposed to oxidative stressors, their red blood cells become more vulnerable to damage. Oxidative stress occurs when there is an imbalance between the production of harmful reactive oxygen species (ROS) and the body’s ability to neutralize them.
- Hemoglobin Denaturation: Oxidative stress leads to the denaturation (unfolding or alteration) of hemoglobin molecules within the red blood cells. Hemoglobin is the protein responsible for carrying oxygen in red blood cells. Denatured hemoglobin forms abnormal clusters within the cells.
- Formation of Heinz Bodies: These abnormal hemoglobin clusters are known as Heinz bodies. Heinz bodies are clumps of denatured hemoglobin that accumulate in the red blood cells. They weaken the cell membrane and reduce its flexibility.
- Spleen’s Role: The spleen is an organ responsible for filtering and removing damaged or abnormal red blood cells from circulation. When the spleen encounters red blood cells containing Heinz bodies, it identifies them as abnormal and targets them for removal.
- Bite Cell Formation: As the spleen removes a red blood cell containing Heinz bodies, it often removes a portion of the cell membrane along with the Heinz body. This selective removal of the membrane results in a characteristic “bite” or notch along the edge of the red blood cell. These cells are termed “bite cells” due to their appearance.
Diagnosis of G6PD Deficiency:
Here are the key steps in the diagnosis of G6PD deficiency.
Clinical Evaluation:
- The diagnostic process often begins with a thorough clinical evaluation by a healthcare provider. They will take a detailed medical history, including any family history of G6PD deficiency or hemolytic anemia.
- The healthcare provider will inquire about any symptoms or signs of anemia, such as fatigue, pale skin, jaundice (yellowing of the skin and eyes), dark urine, and abdominal pain.
- Information about recent exposure to potential triggers of oxidative stress, such as specific medications or foods, is essential.
Laboratory Testing:
- Laboratory tests are crucial for confirming the diagnosis of G6PD deficiency. The primary test used to diagnose G6PD deficiency is a blood test that measures the activity of the G6PD enzyme.
Peripheral Blood Smear:
- A peripheral blood smear examination can be performed to assess the morphology of red blood cells. In cases of G6PD deficiency, characteristic features such as bite cells (notched or bitten red blood cells) may be observed.
G6PD Enzyme Activity Assay:
- The most common diagnostic test for G6PD deficiency is an enzymatic assay to measure G6PD enzyme activity in red blood cells. This test evaluates how effectively the G6PD enzyme is functioning.
- Blood samples are typically collected, and the activity of the G6PD enzyme is measured in the laboratory. Results are reported as a percentage of normal enzyme activity.
- A low enzyme activity level is indicative of G6PD deficiency. However, the severity of the deficiency can vary.
Confirmation and Severity Assessment:
- The diagnosis may be further confirmed by repeating the G6PD enzyme activity assay to ensure accuracy.
- The severity of G6PD deficiency can vary, and laboratory results can help classify it as mild, moderate, or severe.
Genetic Testing (Optional):
- In some cases, genetic testing may be recommended to identify specific mutations in the G6PD gene. Genetic testing can confirm the genetic basis of the deficiency and provide information about the specific G6PD variant involved.
- Genetic testing can be particularly useful for carriers of G6PD deficiency who do not exhibit symptoms but may pass the condition on to their offspring.
Management and Counseling:
- Once the diagnosis is confirmed, individuals with G6PD deficiency should receive counseling and education on avoiding triggers that can lead to hemolysis (destruction of red blood cells).
- Healthcare providers will provide guidance on medications and foods to avoid, especially those known to cause oxidative stress.
Clinical Significance:
Here are some key aspects of its clinical significance.
Hemolytic Anemia:
Hemolytic anemia can manifest with various symptoms and complications, including:
- Fatigue: Due to reduced oxygen-carrying capacity of the blood.
- Pale Skin: Anemia can result in paleness.
- Jaundice: Hemolysis leads to the release of bilirubin, causing yellowing of the skin and eyes.
- Dark Urine: Increased bilirubin can lead to dark-colored urine.
- Enlarged Spleen (Splenomegaly): The spleen may work harder to remove damaged red blood cells.
Symptoms and Triggers:
The clinical significance extends to recognizing and managing symptoms and potential triggers of hemolysis in individuals with G6PD deficiency. Common triggers include.
- Certain medications, such as antimalarials (e.g., primaquine), sulfonamides, and some antibiotics.
- Foods or substances, such as fava beans and naphthalene (found in mothballs).
- Infections, especially those associated with oxidative stress.
- Environmental factors, like exposure to certain chemicals or toxins.
- Varying Severity: The clinical significance of G6PD deficiency can vary widely among individuals. Some individuals may have mild forms of the deficiency with infrequent or mild hemolytic episodes. Others may have moderate to severe forms with more frequent and severe episodes.
- Management: Recognizing the clinical significance of G6PD deficiency is crucial for management. Healthcare providers provide guidance on avoiding triggers, adjusting medications when necessary, and monitoring the patient’s condition. Management also involves addressing complications associated with anemia, such as jaundice and fatigue.
- Genetic Considerations: G6PD deficiency is an inherited condition, and understanding its clinical significance can be important for family planning and genetic counseling. Carriers (heterozygous individuals) may not exhibit symptoms but can pass the condition on to their offspring.
- Prevention and Education: Education about the clinical significance of G6PD deficiency is vital for patients and their families. Awareness of potential triggers and the importance of avoiding them can help prevent hemolytic episodes and associated complications.
Management and Treatment:
Here are key aspects of managing and treating G6PD deficiency.
Trigger Avoidance:
- The cornerstone of managing G6PD deficiency is avoiding known triggers that can induce oxidative stress and lead to hemolysis. Common triggers include specific medications, foods, infections, and chemicals.
- Medications: Healthcare providers should be aware of the patient’s G6PD status when prescribing medications. Individuals with G6PD deficiency should avoid drugs known to cause hemolysis, such as certain antimalarials (e.g., primaquine), sulfonamides, and some antibiotics.
- Foods: Some individuals may experience hemolysis after consuming certain foods or substances, such as fava beans. Dietary restrictions may be recommended.
- Infections: Infections that cause oxidative stress can trigger hemolysis. Prompt treatment of infections is important to minimize the risk.
- Environmental Exposure: Avoiding exposure to chemicals or toxins that can cause oxidative stress is essential. For example, contact with naphthalene (found in mothballs) should be avoided.
Medical Monitoring:
- Individuals with G6PD deficiency should have regular medical check-ups to monitor their overall health and red blood cell status.
- Blood tests, including measurements of hemoglobin levels, reticulocyte counts (immature red blood cells), and bilirubin levels, can provide valuable information about the degree of hemolysis and its impact on the body.
Treatment of Hemolytic Episodes:
- In cases where hemolysis occurs, treatment may be necessary. Treatment options may include blood transfusions to replace the damaged red blood cells and manage severe anemia.
- Supportive care, such as intravenous fluids and pain management, may also be provided during hemolytic episodes.
Genetic Counseling:
- Genetic counseling is recommended for individuals with G6PD deficiency and their families. It can provide information about the inheritance pattern of the condition and the risk of passing it on to future generations.
- Genetic counseling can also assist couples in making informed decisions regarding family planning.
Education:
- Patient and family education is crucial for understanding the condition, recognizing symptoms of hemolysis, and avoiding triggers.
- Individuals with G6PD deficiency should carry a medical alert card or wear a medical alert bracelet to inform healthcare providers about their condition in case of emergencies.
Lifestyle Management:
- Maintaining a generally healthy lifestyle is important for individuals with G6PD deficiency. This includes eating a balanced diet, staying hydrated, and avoiding excessive alcohol consumption.
- Staying informed about potential trigger sources in the environment, such as household chemicals, is also important.
Medications and Vaccinations:
- When necessary, alternative medications that do not trigger hemolysis should be considered. Healthcare providers can recommend suitable options.
- Routine vaccinations, including those for infections like influenza and pneumonia, are generally safe and important for overall health. Consultation with a healthcare provider is advisable.
FAQs:
What is G6PD deficiency?
G6PD deficiency is a genetic disorder that affects the enzyme glucose-6-phosphate dehydrogenase, which plays a crucial role in protecting red blood cells from oxidative damage.
How is G6PD deficiency inherited?
G6PD deficiency is inherited in an X-linked recessive manner. This means that the gene responsible for G6PD is located on the X chromosome. Females with two X chromosomes can be carriers or affected, while males with one X and one Y chromosome are more likely to be affected if they inherit a defective G6PD gene.
What are the common triggers for hemolysis in G6PD deficiency?
Common triggers include certain medications (e.g., antimalarials, sulfonamides), specific foods (e.g., fava beans), infections, and exposure to chemicals or toxins (e.g., naphthalene in mothballs).
How is G6PD deficiency diagnosed?
Diagnosis involves blood tests to measure G6PD enzyme activity and may include a peripheral blood smear examination to look for characteristic features like bite cells. In some cases, genetic testing can be used to identify specific G6PD gene mutations.
What are the symptoms of G6PD deficiency?
Symptoms can include fatigue, pale skin, jaundice (yellowing of the skin and eyes), dark urine, and abdominal pain. These symptoms can result from hemolytic anemia.
How is G6PD deficiency managed and treated?
Management primarily involves avoiding triggers that can lead to oxidative stress and hemolysis. This includes avoiding specific medications, foods, infections, and chemicals. Treatment during hemolytic episodes may include blood transfusions.
Can individuals with G6PD deficiency live normal lives?
Yes, individuals with G6PD deficiency can lead normal, healthy lives by avoiding triggers and managing the condition. Most individuals with mild to moderate G6PD deficiency do not experience frequent or severe hemolytic episodes.
Is there a cure for G6PD deficiency?
G6PD deficiency is a genetic condition, and there is no cure. Management primarily focuses on trigger avoidance and symptom relief during hemolytic episodes.
Can individuals with G6PD deficiency receive vaccinations and medical treatment?
Yes, individuals with G6PD deficiency can receive vaccinations and medical treatment, but caution is needed when selecting medications to avoid those known to trigger hemolysis. Consultation with a healthcare provider is advisable.
Is genetic counseling recommended for individuals with G6PD deficiency?
Yes, genetic counseling can provide important information about the inheritance pattern of G6PD deficiency and help individuals make informed family planning decisions.
Conclusion:
In conclusion, G6PD deficiency is a genetic disorder that affects the G6PD enzyme, making red blood cells vulnerable to oxidative stress and leading to hemolysis. The clinical significance of this condition lies in its potential to cause hemolytic anemia with symptoms such as fatigue, jaundice, and dark urine. Effective management primarily involves avoiding triggers like specific medications and foods, as well as staying informed about environmental factors, while genetic counseling can help individuals and families understand the genetic basis and inheritance pattern. With proper care and avoidance of triggers, individuals with G6PD deficiency can lead normal lives while minimizing the risk of hemolytic episodes and related complications.
Possible References Used