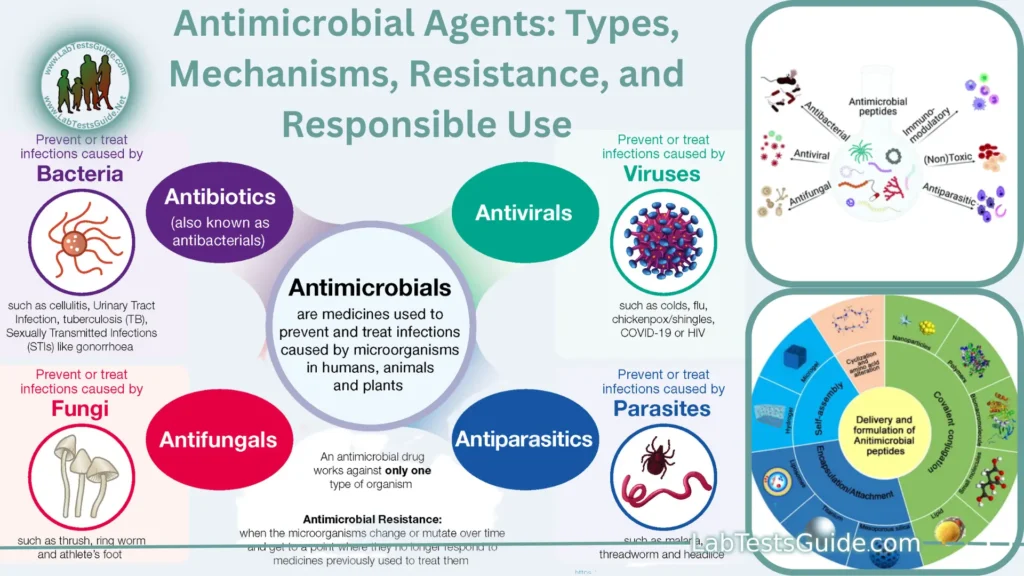
Antimicrobial agents are substances that inhibit or kill microorganisms, including bacteria, viruses, fungi, and parasites. They play a crucial role in medicine and healthcare by helping to treat and prevent infections. Antimicrobials can be categorized into several classes based on their target microorganisms and mechanisms of action
Definition of Antimicrobial Agents.
Antimicrobial agents are substances or compounds that have the ability to kill or inhibit the growth of microorganisms, including bacteria, viruses, fungi, and parasites. These agents are used in various medical, veterinary, and industrial applications to prevent or treat infections caused by these microorganisms.
Types of Antimicrobial Agents:
Here are the main types of antimicrobial agents.
- Antibiotics: Antibiotics are antimicrobial agents that target bacteria. They work by interfering with bacterial processes essential for growth and reproduction. There are several classes of antibiotics, each with its own mechanism of action and spectrum of activity.
Common antibiotic classes include:
- Penicillins
- Cephalosporins
- Tetracyclines
- Macrolides
- Quinolones
- Aminoglycosides
- Antiviral Drugs: Antiviral agents are designed to inhibit the replication and spread of viruses within the host’s cells. They are used to treat viral infections, such as HIV, influenza, herpes, and hepatitis.
Examples of antiviral drugs include:
- Reverse transcriptase inhibitors (used for HIV)
- Neuraminidase inhibitors (used for influenza)
- Nucleoside/nucleotide analogs (used for various viruses)
- Antifungal Drugs: Antifungal agents are used to treat fungal infections, which can affect the skin, nails, mucous membranes, and internal organs. They work by targeting fungal cell structures or functions.
Common antifungal drugs include:
- Azoles
- Polyenes
- Echinocandins
- Allylamines
- Antiparasitic Drugs: These drugs are used to treat infections caused by parasites, such as malaria, amoebiasis, and helminthiasis. They have various mechanisms of action depending on the type of parasite.
Examples of antiparasitic medications include:
- Chloroquine (used for malaria)
- Metronidazole (used for amoebiasis)
- Albendazole (used for helminth infections)
- Antiseptics and Disinfectants: Antiseptics are substances applied to living tissues (e.g., skin and mucous membranes) to reduce microbial growth and prevent infections. Disinfectants are used on surfaces and inanimate objects to kill or inhibit microorganisms.
Common examples include:
- Hydrogen peroxide
- Iodine-based antiseptics
- Bleach (sodium hypochlorite)
- Quaternary ammonium compounds (quats)
- Antimicrobial Peptides: These are naturally occurring molecules produced by the immune system and found in various organisms. They have broad-spectrum activity against bacteria, fungi, and some viruses and are being researched as potential alternatives to traditional antibiotics.
- Phage Therapy: Phage therapy involves the use of bacteriophages (viruses that infect bacteria) to treat bacterial infections. Bacteriophages can specifically target and kill bacterial pathogens.
- Nanotechnology in Antimicrobial Research: Nanoparticles and nanomaterials are being explored for their antimicrobial properties and their potential to combat infections in various applications.
Mechanisms of Action:
some of the common mechanisms of action for different classes of antimicrobial agents.
Antibiotics (Targeting Bacteria):
- Inhibition of Cell Wall Synthesis: Antibiotics like penicillins and cephalosporins disrupt the formation of bacterial cell walls, leading to cell lysis and death.
- Inhibition of Protein Synthesis: Antibiotics such as tetracyclines and macrolides interfere with bacterial ribosomes, preventing protein synthesis.
- Inhibition of Nucleic Acid Synthesis: Quinolones and rifampin inhibit DNA or RNA synthesis in bacteria.
- Disruption of Cell Membranes: Some antibiotics, like polymyxins, disrupt bacterial cell membranes, causing leakage of cellular contents.
- Inhibition of Metabolic Pathways: Sulfonamides and trimethoprim block key metabolic pathways in bacteria, like folate synthesis.
Antiviral Drugs (Targeting Viruses):
- Inhibition of Viral Entry: Entry inhibitors prevent viruses from entering host cells by blocking specific viral attachment or fusion proteins.
- Interference with Viral Replication: Nucleoside analogs, like AZT (zidovudine), interfere with viral DNA or RNA synthesis.
- Protease Inhibition: Protease inhibitors disrupt the cleavage of viral proteins, preventing the maturation of new virus particles.
- Reverse Transcriptase Inhibition: Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) target the reverse transcriptase enzyme in retroviruses like HIV.
- Neuraminidase Inhibition: Neuraminidase inhibitors, such as oseltamivir, inhibit the release of new influenza virus particles from infected cells.
Antifungal Drugs (Targeting Fungi):
- Disruption of Cell Membranes: Polyenes like amphotericin B bind to fungal cell membranes, causing leakage of cellular components.
- Inhibition of Ergosterol Synthesis: Azoles (e.g., fluconazole) interfere with the synthesis of ergosterol, a crucial component of fungal cell membranes.
- Interference with Cell Wall Synthesis: Echinocandins inhibit the synthesis of fungal cell walls, leading to cell lysis.
- Disruption of Microtubules: Griseofulvin disrupts fungal microtubules, inhibiting mitosis.
Antiparasitic Drugs (Targeting Parasites):
- Inhibition of Parasitic Enzymes: Some antiparasitic drugs target specific enzymes in parasites, disrupting their metabolic pathways.
- Disruption of Parasite Membranes: Certain drugs, like chloroquine, interfere with the membranes of parasitic organisms, affecting their survival.
- cInhibition of Parasite Replication: Antiparasitic medications can disrupt the replication cycles of parasites like protozoa and helminths.
Antiseptics and Disinfectants:
- Cell Membrane Disruption: Some antiseptics and disinfectants can damage microbial cell membranes, leading to cell death.
- Protein Denaturation: Chemical agents like heat and certain disinfectants can denature microbial proteins, rendering them nonfunctional.
- Oxidation: Oxidizing agents can damage microbial DNA, proteins, and other cellular components, leading to cell death.
Antimicrobial Resistance:
Here are key aspects of antimicrobial resistance.
Mechanisms of Antimicrobial Resistance:
- Mutation: Microorganisms can undergo genetic mutations that lead to changes in their DNA, enabling them to withstand the effects of antimicrobial agents.
- Horizontal Gene Transfer: Bacteria can transfer resistance genes to one another through mechanisms such as conjugation, transformation, and transduction, allowing resistance traits to spread rapidly within bacterial populations.
- Selective Pressure: Frequent or inappropriate use of antimicrobial agents can exert selective pressure on microorganisms, favoring the survival and proliferation of resistant strains.
Types of Antimicrobial Resistance:
- Bacterial Resistance: Bacteria can develop resistance to antibiotics, which are the most well-known antimicrobial agents. Examples include methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant tuberculosis (MDR-TB).
- Viral Resistance: Viruses can develop resistance to antiviral drugs, particularly in the case of HIV and hepatitis viruses.
- Fungal Resistance: Fungi can become resistant to antifungal medications, posing challenges in treating fungal infections.
- Parasitic Resistance: Parasites, such as Plasmodium species (causing malaria) and some helminths, can develop resistance to antiparasitic drugs.
Consequences of Antimicrobial Resistance:
- Treatment Failures: Infections become more difficult to treat, leading to longer hospital stays, increased healthcare costs, and higher mortality rates.
- Spread of Infections: Resistant microorganisms can spread within healthcare settings, communities, and even across borders, making it harder to control outbreaks.
- Limited Treatment Options: As more microorganisms become resistant, the pool of effective antimicrobial agents diminishes, leaving fewer treatment options.
- Increased Morbidity and Mortality: AMR can result in prolonged illnesses and higher death rates due to infectious diseases.
Factors Contributing to Antimicrobial Resistance:
- Overuse and Misuse: Overprescribing or using antimicrobial agents when they are not needed can accelerate the development of resistance.
- Poor Infection Control: Inadequate hygiene and infection control practices in healthcare settings can lead to the spread of resistant microorganisms.
- Suboptimal Drug Use: Incorrect dosing, incomplete treatment courses, and use of substandard drugs can contribute to resistance.
- Use in Agriculture: The use of antimicrobial agents in agriculture, such as in animal husbandry, can contribute to the development of resistance and may affect food safety.
Strategies to Combat Antimicrobial Resistance:
- Stewardship Programs: Promoting responsible and evidence-based use of antimicrobial agents in healthcare settings.
- Development of New Antimicrobials: Research and development of novel drugs and treatment approaches.
- Improved Diagnostics: Rapid and accurate diagnostic tests can help healthcare providers prescribe the right treatment.
- Enhanced Infection Control: Implementing strict infection prevention and control measures.
- Global Collaboration: International cooperation to address AMR on a global scale.
Responsible Use of Antimicrobials:
Diagnosis and Prescribing:
- Accurate Diagnosis: Ensure that infections are accurately diagnosed through appropriate laboratory tests and clinical assessments before prescribing antimicrobial agents.
- Prescribe Only When Necessary: Antimicrobial agents should be prescribed only when there is clear evidence of a bacterial, viral, fungal, or parasitic infection. They are ineffective against viral infections like the common cold.
Antibiotic Stewardship Programs:
- Implement Stewardship Programs: Healthcare facilities should establish antibiotic stewardship programs to promote the appropriate use of antibiotics. These programs involve dedicated teams monitoring and optimizing antibiotic use.
- Guidelines and Education: Provide healthcare providers with guidelines and education on appropriate antimicrobial prescribing practices.
Proper Dosage and Duration:
- Follow Dosage Guidelines: Ensure that patients are prescribed the correct dosage of antimicrobial agents based on their age, weight, and specific infection.
- Complete Treatment: Encourage patients to complete the full course of treatment, even if their symptoms improve before the medication is finished, to prevent the development of resistance.
Infection Prevention and Control:
- Hand Hygiene: Promote regular handwashing and proper hand hygiene practices among healthcare providers and the public.
- Isolation and Barrier Precautions: Implement isolation and barrier precautions to prevent the spread of resistant infections in healthcare settings.
Surveillance and Monitoring:
- Surveillance of AMR: Regularly monitor and report antimicrobial resistance patterns to identify emerging resistance trends.
- Monitor Treatment Outcomes: Evaluate the effectiveness of treatment regimens and adjust them as needed based on patient responses and laboratory data.
Limit Use in Agriculture:
- Restrict Use in Food Production: Implement policies to limit the use of antimicrobial agents in agriculture and livestock farming to reduce the development and spread of resistance.
Public Education:
- Educate Patients: Educate patients about the appropriate use of antimicrobial agents, including the importance of taking medications as prescribed and not sharing or saving antibiotics.
Research and Development:
- Invest in New Drugs: Support research and development efforts to discover and develop new antimicrobial agents that are less likely to promote resistance.
International Collaboration:
- Global Cooperation: Collaborate with international organizations and other countries to address AMR on a global scale, as resistance can spread across borders.
Regulatory Measures:
- Regulate Antimicrobial Use: Enforce regulations on the sale and distribution of antimicrobial agents to ensure responsible use.
Antiseptics and Disinfectants:
Antiseptics:
- Definition: Antiseptics are antimicrobial agents applied to living tissues (e.g., skin and mucous membranes) to reduce the growth of microorganisms and prevent infections. They are generally safe for use on skin and mucous membranes and are used to disinfect wounds, prepare the skin for medical procedures, and maintain personal hygiene.
Examples of Antiseptics:
- Alcohol: Ethanol and isopropyl alcohol are common antiseptics used for skin disinfection.
- Iodine-Based Antiseptics: Iodine solutions, such as povidone-iodine, are used for wound disinfection and surgical site preparation.
- Hydrogen Peroxide: It is used for wound cleaning and as a mouthwash.
- Chlorhexidine: Used in surgical hand scrubbing, skin disinfection, and oral hygiene products.
- Mechanism of Action: Antiseptics work by disrupting the cell membranes, proteins, and other cellular components of microorganisms, leading to their death or inhibition of growth.
- Applications: Antiseptics are used in healthcare settings, homes, and personal care products to prevent infection in wounds, perform surgical hand hygiene, and ensure general cleanliness and hygiene.
Disinfectants:
- Definition: Disinfectants are chemical agents used to kill or inhibit microorganisms on inanimate objects, surfaces, and environmental settings. They are not typically safe for use on living tissues due to their higher potency.
Examples of Disinfectants:
- Bleach (Sodium Hypochlorite): A strong disinfectant used in various settings, including hospitals and laboratories.
- Quaternary Ammonium Compounds (Quats): These are commonly used in disinfecting surfaces, equipment, and in household cleaning products.
- Phenolic Compounds: Phenol-based disinfectants are effective against a wide range of microorganisms and are used in healthcare facilities.
- Peracetic Acid: Used for disinfection in healthcare settings, including the sterilization of medical equipment.
- Mechanism of Action: Disinfectants work by disrupting cellular structures, proteins, and enzymes in microorganisms, effectively killing them.
- Applications: Disinfectants are used in hospitals, laboratories, food processing facilities, homes, and public places to disinfect surfaces, medical equipment, water supplies, and various items that may harbor or transmit infectious agents.
Key Differences:
- Antiseptics are safe for use on living tissues, while disinfectants are intended for inanimate surfaces and objects.
- Antiseptics are used to prevent infection in wounds, sanitize skin, and maintain personal hygiene, while disinfectants are used to kill or inhibit microorganisms on surfaces and in environmental settings.
- Disinfectants are generally more potent and concentrated than antiseptics due to their intended use on surfaces.
Emerging Trends and Alternatives:
Here are some notable emerging trends and alternatives.
Antimicrobial Peptides (AMPs):
- Definition: AMPs are naturally occurring molecules produced by the immune system and found in various organisms. They have broad-spectrum activity against bacteria, fungi, and some viruses.
- Advantages: AMPs have the potential to serve as alternatives to traditional antibiotics, and their diverse mechanisms of action make it challenging for microbes to develop resistance.
- Applications: Research is ongoing to develop AMP-based therapeutics and antimicrobial coatings for medical devices and surfaces.
Phage Therapy:
- Definition: Phage therapy involves the use of bacteriophages, viruses that infect and kill bacteria, to treat bacterial infections.
- Advantages: Phages are highly specific to their target bacteria, reducing the risk of disrupting the body’s natural microbiota and promoting the evolution of resistance.
- Applications: Phage therapy is being explored as a potential treatment for multidrug-resistant bacterial infections, particularly in cases where antibiotics are ineffective.
Nanotechnology in Antimicrobial Research:
- Definition: Nanotechnology involves the manipulation of materials at the nanoscale. In antimicrobial research, nanoparticles and nanomaterials are used to develop novel antimicrobial agents.
- Advantages: Nanoparticles can be engineered to have unique antimicrobial properties and can penetrate microbial biofilms. They also hold promise for drug delivery and diagnostics.
- Applications: Researchers are developing nanomaterials for antimicrobial coatings, wound dressings, and targeted drug delivery systems.
Combination Therapies:
- Definition: Combination therapies involve using multiple antimicrobial agents, often with different mechanisms of action, to treat infections.
- Advantages: Combining antimicrobial agents can enhance treatment efficacy, reduce the risk of resistance development, and target multiple aspects of microbial biology.
- Applications: Combination therapies are increasingly used in clinical practice, especially for treating drug-resistant infections.
CRISPR-Cas Technologies:
- Definition: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) proteins are used for gene editing and can potentially be used to target and disrupt microbial genes, including those involved in antibiotic resistance.
- Advantages: CRISPR-Cas technologies offer a precise way to target specific genes in microorganisms, potentially preventing the development of resistance.
- Applications: Researchers are exploring CRISPR-Cas technologies for antimicrobial applications, including controlling drug-resistant bacteria.
AI and Machine Learning in Drug Discovery:
- Definition: Artificial intelligence (AI) and machine learning are used to analyze vast datasets and predict potential drug candidates and antimicrobial mechanisms.
- Advantages: These technologies can accelerate drug discovery and identify novel compounds that have antimicrobial properties.
- Applications: AI and machine learning are increasingly integrated into drug discovery processes to identify new antimicrobial agents.
FAQs:
What is antimicrobial resistance (AMR)?
Antimicrobial resistance (AMR) occurs when microorganisms, including bacteria, viruses, fungi, and parasites, adapt and become resistant to the drugs designed to treat them. This makes the standard treatments ineffective and infections persist, increasing the risk of spread to others.
How does antimicrobial resistance happen?
Antimicrobial resistance can develop through genetic changes in microorganisms or through the acquisition of resistance genes. Factors such as overuse or misuse of antimicrobials, incomplete treatment, substandard drugs, and inadequate infection prevention can contribute to its development.
What are the consequences of antimicrobial resistance?
Antimicrobial resistance leads to treatment failures, prolonged illnesses, increased mortality, and higher healthcare costs. It can result in limited treatment options, increased burden on healthcare systems, and the potential for the spread of infections within communities and globally.
How can we combat antimicrobial resistance?
Strategies to combat AMR include responsible use of antimicrobials, public education, development of new antimicrobials, enhanced infection prevention and control, global collaboration, and promoting One Health initiatives that consider human, animal, and environmental health.
What are antiseptics and disinfectants?
Antiseptics are substances that inhibit the growth of microorganisms on living tissues (e.g., skin), while disinfectants are used on inanimate objects and surfaces to kill or reduce microorganisms. Both help prevent infections and maintain cleanliness in various settings.
What are some emerging alternatives to traditional antibiotics?
Emerging alternatives include antimicrobial peptides (AMPs), phage therapy, nanotechnology-based antimicrobials, combination therapies, CRISPR-Cas technologies, and artificial intelligence (AI) and machine learning for drug discovery.
How can I contribute to responsible antimicrobial use?
You can contribute by taking antibiotics only when prescribed by a healthcare professional, completing the full course of treatment, not sharing or saving antibiotics, practicing good hygiene, following vaccination schedules, and supporting public health initiatives that raise awareness about responsible antimicrobial use.
Conclusion:
In conclusion, the ongoing battle against antimicrobial resistance (AMR) presents a critical global challenge that demands immediate attention and comprehensive action. As microorganisms continue to evolve and develop resistance to existing treatments, responsible antimicrobial use, enhanced infection prevention, and the development of innovative therapies must remain at the forefront of our efforts. The future of AMR hinges on global collaboration, research into new antimicrobial agents, improved diagnostics, public awareness, and a commitment to the One Health approach, where human, animal, and environmental health are considered together to safeguard the efficacy of these essential tools in healthcare and disease control. Failure to address AMR could undermine decades of medical progress and threaten the foundations of modern healthcare.
Possible References Used