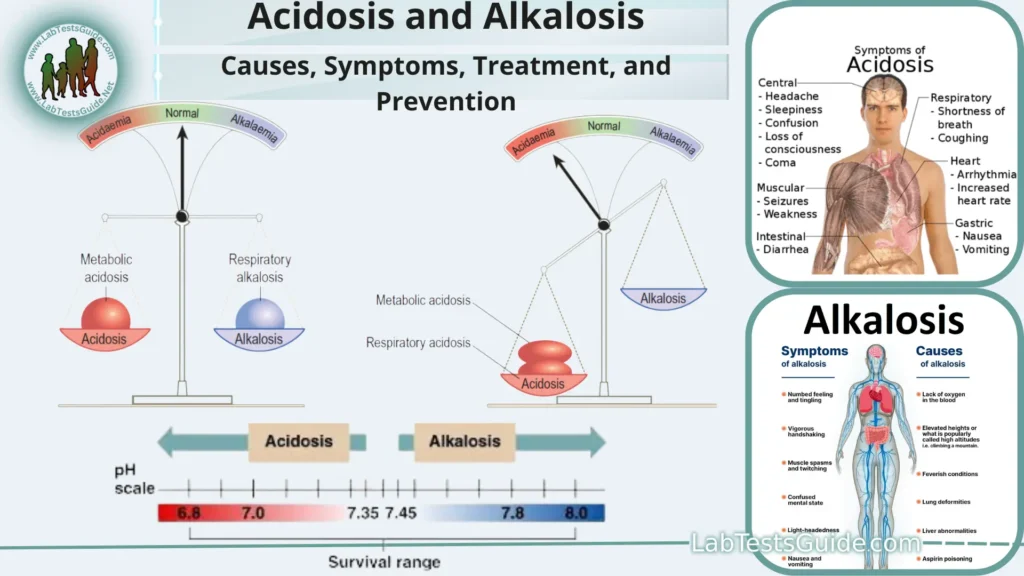
Acidosis and alkalosis are medical conditions characterized by imbalances in the body’s pH levels, which measure the acidity or alkalinity of bodily fluids. The body tightly regulates its pH to maintain a stable and optimal environment for various biochemical processes. The pH scale ranges from 0 (highly acidic) to 14 (highly alkaline), with 7 considered neutral.
Definition of pH.
pH stands for “potential of hydrogen,” and it is a measure of the acidity or alkalinity of a solution. The pH scale is a logarithmic scale that ranges from 0 to 14, with 7 considered neutral.
pH 0-6: Solutions with a pH below 7 are considered acidic. A lower pH indicates greater acidity, with pH 0 being highly acidic.
pH 7: A pH of 7 is considered neutral, meaning the solution is neither acidic nor alkaline. Pure water at 25°C (77°F) is often used as a reference point for neutrality.
pH 8-14: Solutions with a pH above 7 are considered alkaline (basic). A higher pH indicates greater alkalinity, with pH 14 being highly alkaline.
What is Importance of pH balance in the body?
- Enzyme Function: Many enzymes in the body are highly sensitive to changes in pH. Enzymes are biological catalysts that facilitate chemical reactions in the body. They often have specific pH ranges at which they function optimally. Deviations from these pH ranges can inhibit enzyme activity, affecting essential metabolic processes.
- Cellular Function: The pH of the intracellular and extracellular fluids directly impacts the functioning of cells. Cells rely on specific pH levels to maintain proper electrochemical gradients, which are essential for various processes, including the transport of nutrients and ions across cell membranes.
- Blood pH Regulation: The body tightly regulates the pH of the blood to keep it within a narrow range, typically around pH 7.35 to 7.45. This is critical for the functioning of enzymes and proteins in the blood, as well as for oxygen transport by hemoglobin. Deviations from this range can lead to severe health issues, such as acidosis (low blood pH) or alkalosis (high blood pH).
- Respiratory Function: pH balance is intimately tied to the respiratory system. The body uses a process known as acid-base homeostasis to regulate pH by adjusting the rate and depth of breathing. For example, when blood becomes too acidic, the body increases ventilation to expel excess carbon dioxide (a source of acidity). Conversely, when blood becomes too alkaline, ventilation is decreased to retain carbon dioxide.
- Digestive Health: The stomach maintains an acidic environment (low pH) to aid in the digestion of food and kill ingested pathogens. The acidity of stomach acid (gastric acid) is crucial for breaking down proteins and activating digestive enzymes. Imbalances in stomach pH can lead to digestive problems.
- Immune Function: Some components of the immune system, such as white blood cells, function optimally within specific pH ranges. Proper pH levels in bodily fluids are essential for the immune system’s ability to combat infections and diseases.
- Bone Health: The body may draw upon alkaline reserves, such as calcium, from bones to help buffer excess acidity in the blood. Prolonged disturbances in pH balance can lead to bone demineralization and weaken the skeletal system.
- Overall Homeostasis: Maintaining proper pH levels is a critical component of overall homeostasis in the body. Homeostasis refers to the body’s ability to regulate its internal environment and keep it stable. pH balance is intertwined with the regulation of electrolytes, hormones, and various physiological processes.
Acidosis:
Acidosis can have several causes and may occur in two main forms.
Respiratory Acidosis:
This type of acidosis results from a buildup of carbon dioxide (CO2) in the bloodstream due to inadequate removal by the lungs. Normally, the respiratory system helps regulate the body’s pH by eliminating excess CO2. In cases of respiratory acidosis, this elimination process is impaired.
Common causes include:
- Chronic obstructive pulmonary disease (COPD): Conditions like emphysema and chronic bronchitis can reduce the lung’s ability to expel CO2 effectively.
- Hypoventilation: Slow or shallow breathing, which can occur due to certain medications, sedatives, or neuromuscular disorders.
- Airway obstruction: Conditions that restrict airflow, such as asthma or obstructive sleep apnea, can lead to CO2 buildup.
Metabolic Acidosis:
Metabolic acidosis occurs when there is an increase in the production of acid in the body, a decrease in bicarbonate levels, or impaired excretion of acids by the kidneys.
This type of acidosis can be caused by various underlying conditions and factors, including:
- Kidney disease: Kidneys play a vital role in maintaining acid-base balance by excreting excess acids. Kidney dysfunction can lead to metabolic acidosis.
- Diabetic ketoacidosis (DKA): A complication of uncontrolled diabetes, DKA results from the production of acidic ketones when the body cannot use glucose for energy.
- Lactic acidosis: Elevated levels of lactic acid can occur due to conditions like sepsis, shock, severe infections, or certain medications.
- Ingestion of toxic substances: Some toxic substances and poisons can produce metabolic acidosis when metabolized in the body.
- Severe diarrhea: Excessive loss of bicarbonate through diarrhea can lead to metabolic acidosis.
- Dehydration: Inadequate fluid intake or excessive fluid loss can disrupt the body’s acid-base balance.
Common symptoms may include:
- Rapid breathing (in respiratory acidosis) or deep, labored breathing (Kussmaul breathing in metabolic acidosis).
- Confusion or altered mental state.
- Fatigue and weakness.
- Headache.
- Nausea and vomiting.
- Muscle twitching or spasms.
- Elevated heart rate.
Alkalosis:
Alkalosis can occur in two primary forms:
Respiratory Alkalosis:
This type of alkalosis results from an excessive loss of carbon dioxide (CO2) from the body, leading to a decrease in the concentration of carbonic acid in the blood. Carbon dioxide is an acidic substance, and its loss causes the blood to become more alkaline.
Common causes of respiratory alkalosis include:
- Hyperventilation: Rapid and deep breathing, often triggered by anxiety, panic attacks, fever, or certain medications, can lead to excessive CO2 exhalation.
- High-altitude environments: Reduced oxygen levels at high altitudes can stimulate increased ventilation and lead to respiratory alkalosis.
- Fever: Elevated body temperature can sometimes result in hyperventilation and alkalosis.
- Central nervous system disorders: Conditions affecting the brain’s control over breathing, such as brain injuries or brain tumors, can lead to abnormal breathing patterns and alkalosis.
Metabolic Alkalosis:
Metabolic alkalosis occurs when there is an excess of bicarbonate (HCO3-) in the blood, which is a base.
This can be caused by various factors, including.
- Excessive vomiting: Repeated vomiting can lead to the loss of stomach acid (hydrochloric acid), causing an increase in blood bicarbonate levels.
- Diuretic use: Certain diuretic medications can lead to the loss of chloride (Cl-) and sodium (Na+) ions in urine, which can result in metabolic alkalosis.
- Excessive intake of alkaline substances: Ingesting large amounts of antacids, baking soda, or alkaline-rich foods can raise blood bicarbonate levels.
- Kidney disorders: Conditions that affect the kidneys’ ability to regulate bicarbonate levels can result in metabolic alkalosis.
- Hypokalemia: Low levels of potassium in the blood can lead to alkalosis.
Symptoms of alkalosis can vary depending on its underlying cause and severity but may include:
- Muscle twitching or spasms.
- Hand tremors.
- Nausea and vomiting.
- Confusion or irritability.
- Numbness or tingling sensations (paresthesias).
- Rapid and shallow breathing (in respiratory alkalosis).
- Irregular heart rhythms (arrhythmias).
Comparison of Acidosis and Alkalosis:
| Aspect | Acidosis | Alkalosis |
| Definition | Excessive acidity in the body fluids. | Excessive alkalinity in the body fluids. |
| pH Range | Below 7.35 (lower pH) | Above 7.45 (higher pH) |
| Types | Respiratory and Metabolic | Respiratory and Metabolic |
| Causes | Lung diseases, kidney dysfunction, diabetes, metabolic disorders, toxic substances, etc. | Hyperventilation, vomiting, diuretic use, kidney disorders, etc. |
| Common Symptoms | Fatigue, confusion, rapid breathing, muscle weakness, headache. | Muscle twitching, hand tremors, numbness or tingling sensations, nausea, irritability. |
| Diagnostic Tests | Arterial blood gas analysis, metabolic panel, anion gap calculation, urinalysis. | Arterial blood gas analysis, metabolic panel anion gap calculation, urinalysis. |
| Treatment | Address underlying cause, fluids, medications | Address underlying cause, fluids, medications |
| Complications | Organ dysfunction, bone health issues, | Electrolyte imbalances, cardiac arrhythmias, |
| Prognosis | Varies depending on type and cause. | Varies depending on type and cause. |
| Prevention Strategies | Manage chronic conditions, balanced diet, medication management, stress reduction. | Manage chronic conditions, hydration, stress reduction, and lifestyle adjustments. |
Diagnosis and Testing:
Here are some of the key diagnostic methods used:
Arterial Blood Gas (ABG) Analysis:
- Purpose: ABG analysis is a crucial test to assess the pH of arterial blood, as well as the levels of oxygen (PaO2) and carbon dioxide (PaCO2). It provides information about the respiratory component of acid-base balance.
- Procedure: A blood sample is typically drawn from an artery, usually from the radial artery in the wrist. The sample is analyzed for pH, PaO2, PaCO2, and bicarbonate (HCO3-) levels.
- Use: ABG results can help differentiate between respiratory acidosis, respiratory alkalosis, and metabolic acidosis or alkalosis. It also provides insights into the compensatory mechanisms the body may be employing.
Basic Metabolic Panel (BMP) or Comprehensive Metabolic Panel (CMP):
- Purpose: These blood panels include measurements of electrolytes, including bicarbonate (HCO3-), potassium (K+), and chloride (Cl-), which are crucial for assessing the metabolic component of acid-base balance.
- Procedure: A blood sample is taken from a vein and analyzed for various blood chemistry parameters.
- Use: BMP or CMP results can help diagnose metabolic acidosis or alkalosis by examining levels of electrolytes and bicarbonate.
Anion Gap Calculation:
- Purpose: The anion gap is calculated using measurements of sodium (Na+), chloride (Cl-), and bicarbonate (HCO3-) in the blood. It helps identify the underlying cause of metabolic acidosis.
- Formula: Anion Gap = (Na+) – [(Cl-) + (HCO3-)]
- Use: A high anion gap suggests the presence of unmeasured anions, which can help diagnose conditions such as lactic acidosis or ketoacidosis.
Urinalysis:
- Purpose: Urinalysis can provide information about the presence of abnormal substances, such as ketones or glucose, which can be associated with certain types of acidosis (e.g., diabetic ketoacidosis).
- Procedure: A urine sample is collected and analyzed for the presence of specific substances, pH, and other parameters.
- Use: Urinalysis can aid in diagnosing the underlying cause of acidosis and help monitor treatment progress.
Imaging Studies:
- Purpose: In some cases, imaging studies like chest X-rays or CT scans may be conducted to identify respiratory conditions contributing to acid-base imbalances, such as pneumonia or lung diseases.
Assessment of Clinical Signs and Symptoms:
- Purpose: Evaluating the patient’s clinical presentation, including symptoms such as rapid breathing, confusion, or muscle twitching, is essential for diagnosing and determining the severity of acidosis or alkalosis.
Treatment Approaches:
Here are the general treatment approaches for these conditions.
Acidosis Treatment:
Respiratory Acidosis:
- Improve Ventilation: If respiratory acidosis is caused by hypoventilation (ineffective breathing), the underlying cause (e.g., lung disease) should be treated. In some cases, assisted ventilation through mechanical ventilation may be necessary.
- Oxygen Therapy: Oxygen supplementation may be administered to improve oxygen levels in the blood.
Metabolic Acidosis:
Treat the Underlying Cause: Identifying and addressing the underlying condition is crucial.
For example:
- In diabetic ketoacidosis (DKA), insulin and intravenous fluids are used to normalize blood glucose and correct ketoacidosis.
- In cases of kidney dysfunction, addressing the kidney problem and managing electrolyte imbalances are essential.
- Fluid Replacement: Intravenous (IV) fluids may be administered to correct dehydration and replenish bicarbonate levels.
- Medications: In some cases, medications like sodium bicarbonate may be given to raise the blood’s bicarbonate concentration and correct acidosis.
Respiratory Alkalosis:
- Address the Underlying Cause: Treat the condition causing hyperventilation, such as anxiety or fever.
- Breathing Techniques: Patients may be taught to slow their breathing and breathe more deeply to reduce respiratory alkalosis.
Metabolic Alkalosis:
- Treat the Underlying Cause: Identify and address the condition or factors contributing to metabolic alkalosis. This may include discontinuing diuretic medications, treating vomiting, or managing kidney disorders.
- Fluid and Electrolyte Replacement: Administer IV fluids and electrolytes to correct imbalances.
- Potassium Replacement: In cases of hypokalemia (low potassium), potassium supplements may be given.
- Monitoring: Regular monitoring of blood pH, electrolytes, and other relevant parameters is essential to track progress and adjust treatment as needed.
- Address Symptoms: Symptomatic relief is an important aspect of treatment. Medications may be prescribed to manage symptoms such as nausea, muscle spasms, or pain.
- Nutritional Support: Proper nutrition is crucial, especially in cases of metabolic acidosis or alkalosis. Diet modifications may be recommended to address specific electrolyte imbalances or nutritional deficiencies.
- Prevent Recurrence: Preventing the recurrence of acidosis or alkalosis often involves managing the underlying condition and making lifestyle or medication adjustments as needed.
- Respiratory Therapy: In cases of respiratory acidosis or alkalosis, respiratory therapy may be recommended to help improve lung function and breathing patterns.
Complications and Prognosis:
Complications of Acidosis:
- Organ Dysfunction: Untreated or severe acidosis can affect the functioning of various organs, particularly the heart, kidneys, and nervous system. It can lead to cardiac arrhythmias, impaired kidney function, and neurological issues, including confusion, seizures, and coma.
- Bone Health: Chronic metabolic acidosis can lead to the demineralization of bones, increasing the risk of osteoporosis and fractures.
- Lethargy and Muscle Weakness: Acidosis can cause fatigue, muscle weakness, and a general sense of lethargy.
- Complications of Underlying Causes: Acidosis often results from underlying medical conditions, such as kidney disease or diabetes. Complications related to these underlying conditions can also affect the overall prognosis.
- Respiratory Complications: In cases of severe respiratory acidosis, respiratory failure and the need for mechanical ventilation can be life-threatening.
Prognosis for Acidosis:
- The prognosis for acidosis varies depending on its type, underlying causes, and how promptly it is diagnosed and treated. Metabolic acidosis due to conditions like diabetic ketoacidosis can be life-threatening if not treated urgently. However, with appropriate medical intervention, many cases of acidosis can be managed effectively.
Complications of Alkalosis:
- Electrolyte Imbalances: Alkalosis, especially metabolic alkalosis, can lead to imbalances in electrolytes, such as low potassium levels (hypokalemia). These imbalances can affect various bodily functions, including muscle and nerve function.
- Muscle Twitching and Tetany: Alkalosis can cause symptoms like muscle twitching and tetany (involuntary muscle contractions), which can be uncomfortable and distressing.
- Cardiac Arrhythmias: Severe alkalosis can disrupt the electrical activity of the heart, leading to arrhythmias (irregular heart rhythms).
- Cerebral Vasoconstriction: In cases of severe alkalosis, the brain’s blood vessels may constrict, potentially leading to neurological symptoms like dizziness, confusion, and seizures.
Prognosis for Alkalosis:
- The prognosis for alkalosis depends on its type, the underlying causes, and the promptness of treatment. Respiratory alkalosis, often triggered by anxiety or fever, typically resolves once the underlying cause is addressed. Metabolic alkalosis can have a favorable prognosis if the underlying condition is treated effectively and electrolyte imbalances are corrected.
Prevention:
Here are some prevention strategies for these acid-base imbalances.
- Manage Chronic Medical Conditions: If you have chronic medical conditions like diabetes, kidney disease, or lung disorders, work closely with your healthcare provider to manage and control these conditions effectively. Proper disease management can help prevent acid-base imbalances.
- Medication Management: If you are taking medications that can potentially disrupt your acid-base balance (e.g., diuretics), take them as prescribed and under the supervision of your healthcare provider. Regular monitoring of blood chemistry can help catch and address imbalances early.
- Stay Hydrated: Adequate hydration is essential to maintain proper bodily functions. Ensure you drink enough water daily, especially in hot weather or when engaging in physical activity.
- Balanced Diet: Consume a balanced diet that provides essential nutrients and electrolytes. In cases of metabolic acidosis or alkalosis, dietary adjustments may be recommended, such as increasing potassium-rich foods or reducing foods high in bicarbonate.
- Avoid Excessive Antacid Use: If you suffer from conditions like heartburn or acid reflux, be cautious with the use of antacids. Overuse of antacids containing bicarbonate can contribute to metabolic alkalosis. Follow your healthcare provider’s recommendations for managing acid reflux.
- Stress Management: Stress and anxiety can trigger hyperventilation, leading to respiratory alkalosis. Practice stress management techniques like deep breathing, relaxation exercises, or mindfulness to reduce anxiety.
- Be Aware of Altitude Changes: If you travel to high-altitude locations, be aware of the potential for respiratory alkalosis due to changes in oxygen levels. Take time to acclimatize to the altitude, and if necessary, use supplemental oxygen.
- Avoid Toxic Substances: Be cautious about exposure to toxic substances that can lead to metabolic acidosis, such as certain chemicals or substances that can damage the kidneys or liver.
- Regular Check-ups: Attend regular health check-ups, especially if you have underlying medical conditions that could predispose you to acidosis or alkalosis. Early detection and management of these conditions can help prevent imbalances.
- Medication Adjustments: If you experience side effects or symptoms that may be related to medication use (e.g., vomiting or diarrhea due to a medication), consult your healthcare provider. They may need to adjust your medication regimen to avoid potential acid-base imbalances.
- Seek Medical Attention Promptly: If you experience symptoms such as persistent vomiting, severe diarrhea, unexplained muscle spasms, confusion, or difficulty breathing, seek prompt medical attention. Early intervention can prevent the progression of acidosis or alkalosis.
Differences Beween Acidosis and Alkalosis:
Acidosis and alkalosis are two distinct conditions that refer to imbalances in the body’s acid-base balance, primarily related to the pH level of bodily fluids. Here are 15 key differences between acidosis and alkalosis:
- Definition:
- Acidosis: It occurs when the pH of the blood and body tissues drops below the normal range (usually below 7.35).
- Alkalosis: It occurs when the pH of the blood and body tissues rises above the normal range (usually above 7.45).
- Causes:
- Acidosis: Common causes include excessive production of acids, kidney dysfunction, respiratory issues, and certain diseases.
- Alkalosis: Common causes include excessive loss of acids, hyperventilation, certain medications, and electrolyte imbalances.
- pH Levels:
- Acidosis: pH levels below 7.35.
- Alkalosis: pH levels above 7.45.
- Respiratory vs. Metabolic:
- Acidosis: Can be classified as respiratory acidosis (related to impaired lung function) or metabolic acidosis (related to issues with metabolism or kidney function).
- Alkalosis: Can be classified as respiratory alkalosis (due to excessive breathing) or metabolic alkalosis (related to issues with metabolism or kidney function).
- Symptoms:
- Acidosis: Symptoms may include confusion, rapid breathing, fatigue, headache, and muscle weakness.
- Alkalosis: Symptoms may include muscle twitching, tingling sensations, dizziness, nausea, and confusion.
- Compensation Mechanism:
- Acidosis: The body compensates by increasing respiratory rate to eliminate excess CO2 (carbon dioxide), which is acidic.
- Alkalosis: The body compensates by decreasing respiratory rate to retain more CO2.
- Blood Gases:
- Acidosis: Blood gases typically show low pH, high PaCO2 (partial pressure of carbon dioxide), and normal or low bicarbonate (HCO3) levels.
- Alkalosis: Blood gases typically show high pH, low PaCO2, and normal or high bicarbonate levels.
- Common Conditions:
- Acidosis: Conditions such as diabetic ketoacidosis, renal failure, and severe diarrhea can lead to acidosis.
- Alkalosis: Conditions such as hyperventilation, vomiting, and excessive use of antacids can lead to alkalosis.
- Treatment:
- Acidosis: Treatment involves addressing the underlying cause, administering bicarbonate in some cases, and improving lung or kidney function.
- Alkalosis: Treatment involves addressing the underlying cause, slowing down respiratory rate in the case of respiratory alkalosis, and correcting electrolyte imbalances.
- Potential Complications:
- Acidosis: Severe acidosis can lead to coma, arrhythmias, and organ failure.
- Alkalosis: Severe alkalosis can cause seizures, hypokalemia (low potassium levels), and cardiac arrhythmias.
- pH Regulation:
- Acidosis: Associated with an excess of hydrogen ions (H+).
- Alkalosis: Associated with a deficit of hydrogen ions (H+).
- Breathing Patterns:
- Acidosis: May lead to Kussmaul breathing (deep, labored breathing) to compensate for increased CO2.
- Alkalosis: Often associated with rapid, shallow breathing.
- Electrolyte Abnormalities:
- Acidosis: Can cause hyperkalemia (high potassium levels).
- Alkalosis: Can cause hypokalemia (low potassium levels).
- Diagnosis:
- Acidosis: Diagnosed based on arterial blood gas analysis and clinical symptoms.
- Alkalosis: Diagnosed similarly through blood gas analysis and clinical assessment.
- Chronic vs. Acute:
- Acidosis: Can be chronic (long-term) or acute (sudden onset).
- Alkalosis: Can also be chronic or acute, depending on the underlying cause.
Similarities Between Acidosis and Alkalosis:
While acidosis and alkalosis are opposite conditions related to the body’s acid-base balance, there are some similarities between them, particularly in terms of how they are diagnosed and treated. Here are 15 similarities between acidosis and alkalosis:
- Diagnostic Tests: Both acidosis and alkalosis are diagnosed using similar diagnostic tests, including arterial blood gas analysis, which measures the pH, PaCO2 (partial pressure of carbon dioxide), and bicarbonate (HCO3) levels in the blood.
- pH Deviation: In both conditions, there is a deviation from the normal pH range of 7.35 to 7.45, although in opposite directions.
- Respiratory and Metabolic Forms: Both acidosis and alkalosis can manifest as either respiratory or metabolic, depending on whether the primary cause is related to the lungs or metabolism/kidneys.
- Compensation Mechanisms: The body employs compensation mechanisms in both conditions to maintain acid-base balance. In acidosis, the respiratory system compensates by adjusting the breathing rate, and in alkalosis, it does the same to maintain CO2 levels.
- Clinical Symptoms: Acidosis and alkalosis can both present with clinical symptoms, including neurological changes, muscle weakness, and changes in respiratory rate.
- Underlying Causes: While the specific causes differ, both conditions can result from various underlying medical conditions, medications, or lifestyle factors.
- Treatment Principles: Treatment strategies for acidosis and alkalosis focus on addressing the underlying cause. This often involves addressing issues related to the lungs, kidneys, or metabolism.
- Electrolyte Imbalances: Both conditions can lead to electrolyte imbalances, such as hyperkalemia (high potassium levels) in acidosis and hypokalemia (low potassium levels) in alkalosis.
- Fluid and Electrolyte Replacement: In severe cases of acidosis or alkalosis, treatment may involve the administration of fluids and electrolytes to correct imbalances and restore normal pH levels.
- Medical Evaluation: Diagnosis and management of both conditions require a thorough medical evaluation, including a patient’s medical history and a physical examination.
- Acid-Base Homeostasis: Acidosis and alkalosis both disrupt the body’s acid-base homeostasis, which can have serious health consequences if not corrected.
- Respiratory Rate: Changes in respiratory rate play a crucial role in both conditions, as the body tries to compensate by adjusting CO2 levels through altered breathing patterns.
- Neurological Effects: Both conditions can affect the nervous system, leading to symptoms such as confusion, dizziness, and altered mental status.
- Monitoring: Patients with acidosis and alkalosis may require continuous monitoring of vital signs, blood gas levels, and electrolytes to assess their response to treatment.
- Potential Complications: Severe cases of acidosis and alkalosis can lead to life-threatening complications, such as cardiac arrhythmias, organ failure, and seizures.
FAQs:
What is the pH scale, and how does it relate to acidosis and alkalosis?
The pH scale measures the acidity or alkalinity of a solution. It ranges from 0 (highly acidic) to 14 (highly alkaline), with 7 considered neutral. In the context of acidosis and alkalosis, pH levels below 7 indicate acidosis, while levels above 7 indicate alkalosis.
What are the common symptoms of acidosis and alkalosis?
Common symptoms of acidosis may include fatigue, confusion, rapid breathing, muscle weakness, and headache. Alkalosis symptoms can include muscle twitching, hand tremors, numbness or tingling sensations, nausea, and irritability.
What are the primary types of acidosis and alkalosis, and how do they differ?
Acidosis can be categorized into respiratory acidosis (due to lung dysfunction) and metabolic acidosis (resulting from factors other than the respiratory system). Similarly, alkalosis can be respiratory (related to the respiratory system) or metabolic (due to non-respiratory factors).
What causes respiratory acidosis and alkalosis?
Respiratory acidosis is caused by conditions that impair the removal of carbon dioxide (CO2) from the body, such as lung diseases or hypoventilation. Respiratory alkalosis results from hyperventilation, often triggered by factors like anxiety or fever.
What are some common causes of metabolic acidosis and alkalosis?
Metabolic acidosis can be caused by conditions like kidney disease, diabetic ketoacidosis (DKA), lactic acidosis, and severe diarrhea. Metabolic alkalosis can result from excessive vomiting, diuretic use, excessive intake of alkaline substances, or kidney disorders.
How are acidosis and alkalosis diagnosed?
Diagnosis involves blood tests to measure pH, bicarbonate levels, carbon dioxide levels, and sometimes anion gap calculation. Urinalysis and imaging studies may also be used to identify underlying causes.
What are the treatment approaches for acidosis and alkalosis?
Treatment depends on the type and underlying cause but often involves addressing the root cause, fluid and electrolyte management, medications, and sometimes assisted ventilation for severe cases.
What complications can arise from acidosis and alkalosis?
Complications may include organ dysfunction, electrolyte imbalances, cardiac arrhythmias, and bone health issues. The severity of complications depends on the type and severity of the acid-base imbalance.
How can acidosis and alkalosis be prevented?
Prevention strategies involve managing underlying medical conditions, staying hydrated, balanced nutrition, avoiding excessive antacid use, and stress management. Timely medical attention and medication management are also essential.
What is the prognosis for acidosis and alkalosis?
Prognosis varies depending on the type, severity, and underlying causes. Prompt diagnosis and appropriate treatment typically lead to better outcomes, while severe or untreated cases can have serious consequences.
Conclusion:
In conclusion, acidosis and alkalosis are critical medical conditions characterized by disruptions in the body’s acid-base balance, leading to either excess acidity or alkalinity in the blood and bodily fluids. These imbalances can result from various underlying causes, including respiratory and metabolic factors. Timely diagnosis and treatment, tailored to the specific type and cause of the imbalance, are essential to prevent complications and ensure a favorable prognosis. Maintaining overall health, managing chronic conditions, and seeking prompt medical attention when symptoms arise are key steps in preventing and effectively managing acidosis and alkalosis, thus promoting long-term well-being.
Possible References Used