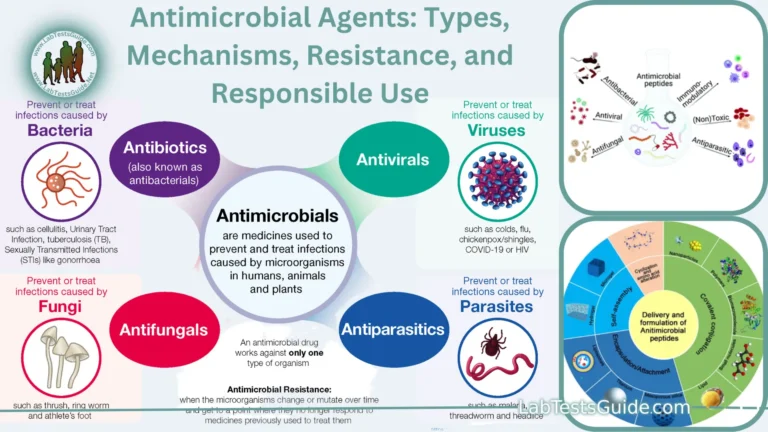
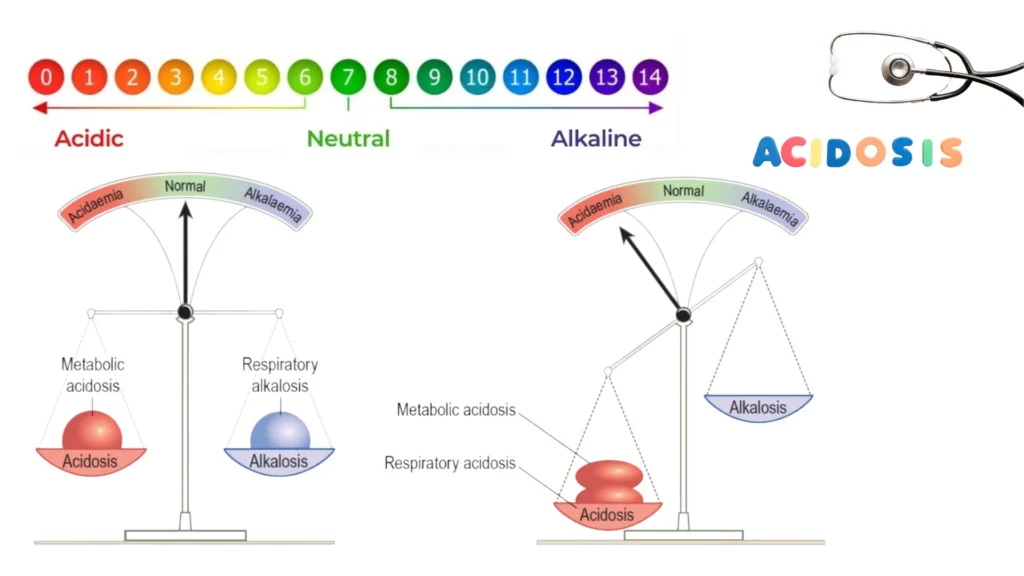
A condition in which there is a change in the body’s acid-base balance to have more acid than normal, which often causes the pH of blood and body tissues to fall below the healthy range (7.35-7.45) . It can be caused by a decrease in the elimination of CO2 in respiratory disorders such as emphysema, by metabolic problems such as kidney disease and diabetes, or as a result of ingestion of poisons (ethylene glycol, methanol) or an overdose of certain medications (salicylates); It can also be caused by loss of HCO3, as in diarrhea.
Understanding Acidosis: A Guide for Clinical Laboratory Professionals
Introduction:
Acidosis is a condition characterized by an excess of acid in the blood, leading to a lower than normal pH. It can result from an overproduction of acid, an underproduction of bicarbonate, or issues with acid excretion. Acidosis is often classified into two types: metabolic and respiratory. Understanding these types is crucial for accurate diagnosis and treatment.
Types of Acidosis:
- Metabolic Acidosis: This occurs when the body produces too much acid or when the kidneys fail to remove enough acid from the body. It can be caused by conditions such as diabetic ketoacidosis, lactic acidosis, and renal failure.
- Respiratory Acidosis: This happens when the lungs cannot remove enough carbon dioxide (CO2) from the body, leading to an accumulation of CO2 in the blood. Conditions like chronic obstructive pulmonary disease (COPD), asthma, and certain neuromuscular diseases can lead to respiratory acidosis.
Laboratory Diagnosis:
Accurate diagnosis of acidosis involves several laboratory tests, including:
- Arterial Blood Gas (ABG) Analysis: Measures the pH, partial pressure of carbon dioxide (PaCO2), and bicarbonate (HCO3-) levels in arterial blood to assess acid-base balance.
- Serum Electrolytes: Includes sodium, potassium, chloride, and bicarbonate. These values can help identify the underlying cause of acidosis.
- Anion Gap Calculation: Helps differentiate between various types of metabolic acidosis. An increased anion gap suggests the presence of unmeasured anions, as seen in conditions like diabetic ketoacidosis or lactic acidosis.
Clinical Implications:
Understanding the type and cause of acidosis is vital for effective treatment. For example, metabolic acidosis may require interventions such as bicarbonate therapy, while respiratory acidosis might need mechanical ventilation or treatment of the underlying pulmonary condition.
Conclusion:
As clinical laboratory professionals, staying informed about acidosis and its implications is essential for accurate diagnosis and patient management. By conducting precise laboratory tests and interpreting the results correctly, we play a critical role in ensuring patients receive appropriate care.
Possible References Used