Acidified silver nitrate solution is a chemical reagent commonly used in clinical laboratories, particularly in diagnostic and research settings. This solution is known for its applications in various analytical and preparative procedures due to its unique properties.
Uses of Acidified Silver Nitrate Solution:
Here are the main uses of acidified silver nitrate solution:
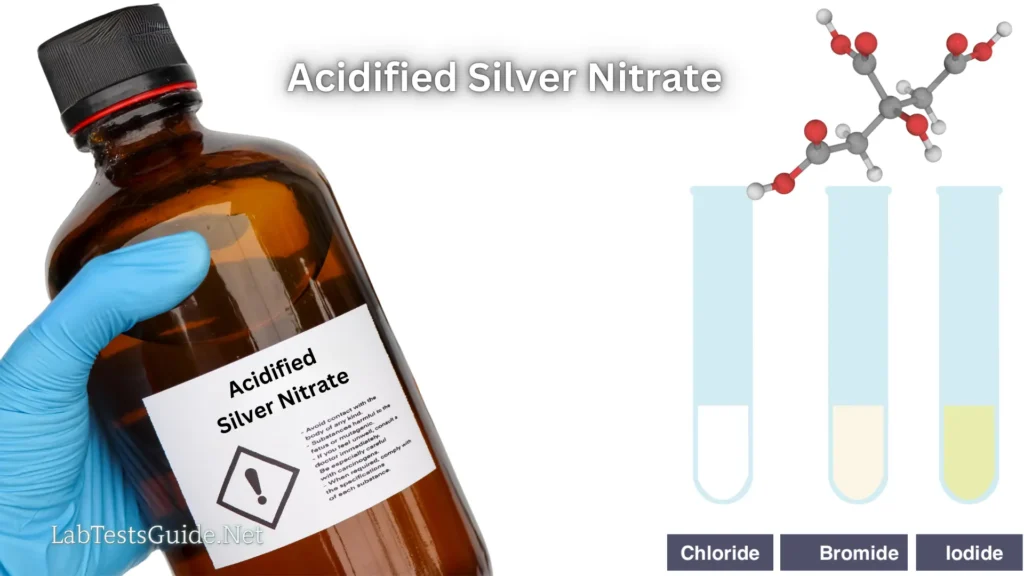
- Detection of Chloride Ions: Used in qualitative analysis to test for the presence of chloride ions in a solution. The reaction forms a white precipitate of silver chloride.
- Testing for Halides: Helps in identifying halide ions (chlorides, bromides, and iodides) by producing distinct precipitates upon reaction with the respective halide ions.
- Photographic Industry: Used in the preparation of light-sensitive materials for photography, where silver salts are crucial.
- Disinfection: Historically used for its antibacterial properties in wound care and in newborn eye prophylaxis to prevent gonococcal infections.
- Analytical Chemistry: Employed in various analytical methods for its reactivity and role in identifying specific ions and compounds.
Composition of Acidified Silver Nitrate Solution:
For preparing 500 mL of acidified silver nitrate solution.
| Component | Amount |
|---|---|
| Silver Nitrate | 14.5 g |
| Nitric Acid (7.7 mol/L) | 250mL (50%) |
| Distilled Water | 250mL (50%) |
- Prepare Nitric Acid Solution: Mix 250 mL of concentrated nitric acid with 250 mL of distilled water. Add the nitric acid to the water.
- Caution: Concentrated nitric acid is a highly corrosive chemical. Handle it with care, using appropriate safety equipment, and work in a well-ventilated area or under a fume hood.
Preparation of Acidified Silver Nitrate Solution:
Here is the brief preparation procedure for acidified silver nitrate solution:
- Weigh and Transfer: Weigh 14.5 g of silver nitrate and transfer it to a 500 mL volumetric flask.
- Caution: Silver nitrate is oxidizing and corrosive; handle with care.
- Dissolve: Half-fill the flask with nitric acid solution (7.7 mol/L), and mix until the silver nitrate is completely dissolved.
- Adjust Volume: Make up the volume to the 500 mL mark with the nitric acid solution (7.7 mol/L), and mix well.
- Caution: Ensure the flask is tightly stoppered. The solution is colorless but can stain skin, clothing, and surfaces.
- Transfer: Move the solution to a dark glass bottle.
- Label: Clearly label the bottle as “Corrosive.”
- Store: Keep at room temperature, away from direct sunlight. The solution remains stable for several months.
Precautions:
- Protective Gear: Wear gloves, safety goggles, and a lab coat.
- Handle with Care: Silver nitrate and nitric acid are corrosive. Use appropriate PPE and handle in a well-ventilated area.
- Add Acid to Water: Always add nitric acid to water to avoid dangerous reactions.
- Avoid Stains: The solution can stain skin and surfaces. Clean spills immediately.
- Proper Storage: Store in a dark glass bottle, label as “Corrosive,” and keep at room temperature away from sunlight.
- Emergency Procedures: Be prepared for spills and have first aid measures ready.
Uses of Acidified Silver Nitrate Solution in Clinical Laboratories:
- Chloride Detection: Identifies chloride ions in samples by forming a white precipitate of silver chloride.
- Halide Testing: Differentiates between chloride, bromide, and iodide ions through precipitation reactions.
- Microscopy: Used in some staining procedures to visualize specific cellular components.
- Tissue Preparation: Applied in histology for silver staining to highlight certain cellular structures and proteins.
- Antimicrobial Applications: Historically used for its antimicrobial properties in wound care.
Possible References Used