MacConkey Sorbitol Agar (SMAC) is a specialized culture medium for detecting sorbitol-nonfermenting E. coli serotype O157:H7, a pathogen linked to hemorrhagic colitis. While standard MacConkey Agar can’t distinguish this strain from others, E. coli O157:H7 exhibits slow or no sorbitol fermentation. Sorbitol MacConkey Agar, with added cefixime and tellurite, selectively inhibits unrelated bacteria like Proteus mirabilis, improving isolation of E. coli O157:H7 in clinical and environmental samples.
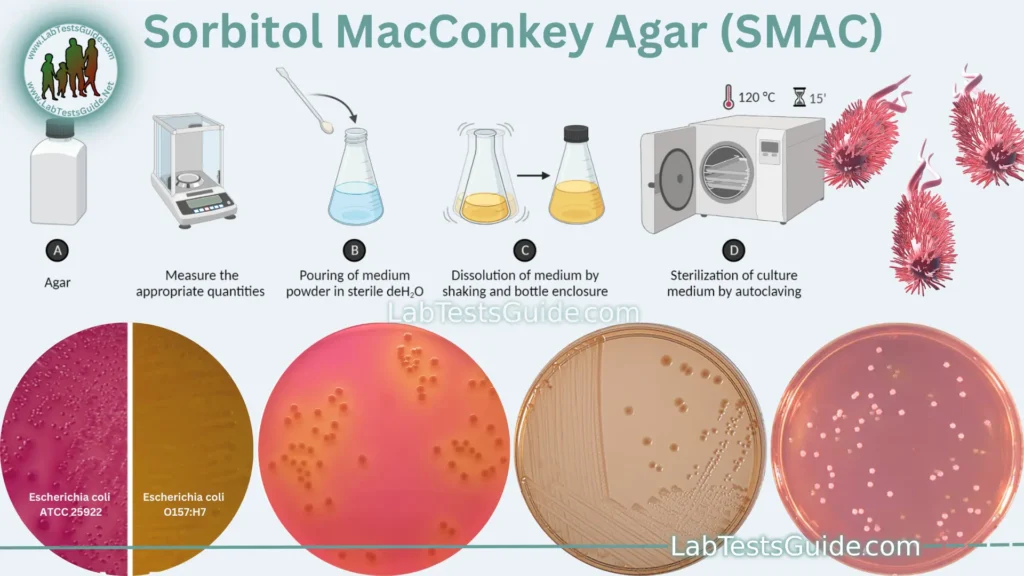
Key Points of Sorbitol MacConkey Agar:
- Purpose: Sorbitol MacConkey Agar is a specialized microbiological culture medium.
- Variant of MacConkey Agar: It is a variant of MacConkey Agar, specifically designed to isolate and differentiate certain bacterial strains.
- Selective Medium: It is selective for gram-negative bacteria.
- Lactose as a Carbohydrate Source: Like MacConkey Agar, it contains lactose as a primary carbohydrate source.
- Sorbitol Inclusion: It differs from standard MacConkey Agar by the inclusion of sorbitol.
- Sorbitol as a Sugar Alcohol: Sorbitol is a sugar alcohol that some bacteria can ferment.
- E. coli Differentiation: Sorbitol MacConkey Agar is primarily used to differentiate between different strains of Escherichia coli (E. coli).
- E. coli O157:H7: This medium is crucial for identifying E. coli O157:H7, a pathogenic strain.
- Sorbitol Fermentation: Non-pathogenic E. coli strains ferment sorbitol, while pathogenic E. coli O157:H7 does not.
- Acid Production: Fermentation of sorbitol or lactose leads to the production of acids.
- Neutral Red Indicator: It contains a pH indicator, Neutral Red, which changes color in response to pH changes.
- pH Indicator Interpretation: Acid production turns the medium from red to colorless or pale.
- Pink/Red Colonies: E. coli strains that ferment sorbitol or lactose appear as pink or red colonies.
- Colorless Colonies: E. coli O157:H7 appears as colorless or pale colonies due to its inability to ferment sorbitol.
- Inhibition of Gram-Positives: Sorbitol MacConkey Agar inhibits the growth of gram-positive bacteria.
- Diagnostic Tool: It is used in clinical microbiology for the diagnosis of E. coli O157:H7 infections.
- Food Safety: Used in food microbiology to detect E. coli O157:H7 in food samples.
- Research Applications: Applied in research settings to study bacterial characteristics and pathogenicity.
- Culture Conditions: Bacterial cultures are grown on solidified agar plates.
- Incubation Temperature: Typically incubated at 35-37°C.
- Incubation Time: Plates are examined after 24-48 hours of incubation.
- Colonial Morphology: Helps differentiate between bacterial species based on colony appearance.
- Laboratory Safety: Proper laboratory safety procedures should be followed when working with microbiological cultures.
- Confirmation Tests: Further tests like biochemical and serological assays may be used to confirm the identity of bacterial isolates.
Defination of Sorbitol MacConkey Agar:
Sorbitol MacConkey Agar is a selective and differential microbiological medium used to identify sorbitol-nonfermenting Escherichia coli serotype O157:H7, a pathogen associated with hemorrhagic colitis, by detecting its unique sorbitol fermentation characteristics.
History and Modifications of SMAC Agar:
Sorbitol MacConkey Agar (SMAC) has its origins in the MacConkey Agar, which was developed by Alfred Theodore MacConkey in the late 19th century. MacConkey Agar was designed to selectively isolate and differentiate lactose-fermenting gram-negative bacteria, particularly Enterobacteriaceae, by incorporating lactose and bile salts into the medium.
The modification of MacConkey Agar to create Sorbitol MacConkey Agar occurred in the late 20th century, specifically for the detection of Escherichia coli serotype O157:H7, a virulent strain associated with hemorrhagic colitis and hemolytic uremic syndrome.
Here’s a brief history and key modifications:
- Development of MacConkey Agar: Alfred MacConkey initially developed MacConkey Agar in the late 19th century as a selective medium to differentiate lactose-fermenting bacteria from non-lactose fermenters. The medium incorporated lactose and bile salts, which inhibited the growth of gram-positive bacteria and allowed for the growth of gram-negative bacteria.
- Identification of E. coli O157:H7: In the 1980s, as the recognition of E. coli O157:H7 as a significant human pathogen grew, there was a need for a selective medium to distinguish it from other E. coli strains. It was noted that E. coli O157:H7 did not ferment sorbitol like most other E. coli strains.
- Sorbitol Modification: To achieve this selectivity, sorbitol was incorporated into MacConkey Agar. This modification allowed for the differentiation of E. coli O157:H7, which either ferments sorbitol slowly or not at all, from other E. coli strains.
- Inhibition of Unrelated Bacteria: To enhance the medium’s selectivity for E. coli O157:H7, cefixime and tellurite were added. These compounds inhibit the growth of unrelated bacteria, such as Proteus mirabilis and other sorbitol-nonfermenting strains.
- Clinical Significance: The modified Sorbitol MacConkey Agar has played a crucial role in the clinical identification of E. coli O157:H7, which is responsible for foodborne outbreaks and gastrointestinal illnesses. Its ability to specifically detect this pathogen has been instrumental in public health and food safety efforts.
Purpose and Significance of SMAC Agar:
The purpose and significance of Sorbitol MacConkey Agar (SMAC) lie in its specific applications in microbiology, particularly in the detection and differentiation of pathogenic Escherichia coli serotype O157:H7. Here’s a closer look at its purpose and significance:
Purpose:
- Identification of E. coli O157:H7: The primary purpose of Sorbitol MacConkey Agar is to identify E. coli serotype O157:H7, a pathogenic strain responsible for severe gastrointestinal illnesses, including hemorrhagic colitis. Unlike most E. coli strains, E. coli O157:H7 does not ferment sorbitol efficiently or at all.
- Distinguishing Pathogenic Strains: SMAC is used to distinguish E. coli O157:H7 from other non-pathogenic E. coli strains that do ferment sorbitol. This differentiation is crucial for public health and epidemiological investigations.
Significance:
- Food Safety: SMAC plays a pivotal role in food safety and epidemiology. It is used to detect E. coli O157:H7 in food samples, helping to identify and control outbreaks of foodborne illnesses. It enables swift action to remove contaminated products from the market.
- Clinical Diagnosis: In clinical microbiology, SMAC is employed to isolate and identify E. coli O157:H7 in patients with gastrointestinal infections. Rapid identification aids in the appropriate treatment and management of infected individuals.
- Public Health Surveillance: The ability to distinguish E. coli O157:H7 from other E. coli strains is vital for monitoring and controlling outbreaks in communities, restaurants, and healthcare settings. It aids in tracking the source of contamination.
- Research and Epidemiology: SMAC is used in research to study the prevalence and characteristics of E. coli O157:H7 in various environments, including water sources, agricultural settings, and animal reservoirs. This research informs public health strategies.
- Selective and Differential Medium: SMAC’s selective nature inhibits the growth of unrelated bacteria and other E. coli strains, allowing for the isolation of E. coli O157:H7 with minimal interference.
- Human Health Protection: The significance of SMAC lies in its role in protecting human health by aiding in the rapid identification and containment of a bacterial pathogen associated with severe illnesses. This is particularly important in preventing outbreaks and ensuring timely medical intervention.
Importance of SMAC Agar in Microbiology:
- Identification of E. coli O157:H7: It enables the specific detection and differentiation of pathogenic E. coli serotype O157:H7.
- Food Safety: Critical for identifying E. coli O157:H7 in food samples, ensuring safe consumption.
- Clinical Diagnosis: Used in clinical microbiology to isolate and diagnose E. coli O157:H7 infections.
- Outbreak Control: Helps track and control outbreaks in public health settings.
- Research Tool: Used in research to study the prevalence and characteristics of E. coli O157:H7.
- Selective Medium: Its selectivity inhibits unrelated bacteria, facilitating pure culture isolation.
- Human Health Protection: Vital for timely intervention and prevention of severe gastrointestinal illnesses.
Short: E. coli serotype O157:H7:
E. coli serotype O157:H7, often referred to as E. coli O157:H7, is a pathogenic strain of Escherichia coli bacteria. It is known for its association with severe gastrointestinal illnesses in humans. Here’s a short overview:
- Pathogenic Strain: E. coli O157:H7 is a virulent strain of E. coli, capable of causing illness in humans.
- Gastrointestinal Infections: It is a leading cause of foodborne illnesses and is associated with conditions such as hemorrhagic colitis and hemolytic uremic syndrome (HUS).
- Shiga-Like Toxin Production: E. coli O157:H7 produces Shiga-like toxins (SLTs), which can lead to serious complications, including kidney damage.
- Symptoms: Infections may result in symptoms such as diarrhea (often bloody), abdominal pain, vomiting, and fever.
- Transmission: It is typically transmitted through contaminated food, water, or contact with infected animals or their environments.
- Importance: E. coli O157:H7 is a public health concern, and its detection and monitoring are crucial for preventing and managing outbreaks.
- Sorbitol Nonfermenter: It distinguishes itself by its inability to efficiently ferment sorbitol, making Sorbitol MacConkey Agar a valuable diagnostic tool for its identification.
- Control Measures: Efforts to prevent E. coli O157:H7 infections include proper food handling, hygiene practices, and surveillance in food production and distribution.
Principles of Sorbitol MacConkey Agar:
The principles of Sorbitol MacConkey Agar (SMAC) are based on its selective and differential properties, which allow for the isolation and identification of specific bacteria, primarily Escherichia coli serotype O157:H7. Here are the key principles of SMAC:
- Selective Medium: SMAC is designed to selectively inhibit the growth of certain bacteria while allowing the growth of others. It does this through the following principles:
- Inhibition of Gram-Positives: The medium contains bile salts and crystal violet, which inhibit the growth of gram-positive bacteria, including most species of staphylococci and streptococci.
- Inhibition of Lactose-Fermenting Gram-Negatives: SMAC inhibits the growth of lactose-fermenting gram-negative bacteria that typically grow on regular MacConkey Agar. This is achieved by incorporating sorbitol as the sole carbohydrate source.
- Differential Medium: SMAC is also a differential medium, allowing for the differentiation of bacterial strains based on their ability to ferment sorbitol and produce acid. This is crucial for the identification of Escherichia coli serotype O157:H7:a. Sorbitol Fermentation: Most E. coli strains, when they ferment sorbitol, produce acid as a metabolic byproduct. On SMAC, these strains appear as pink or red colonies due to the pH indicator (Neutral Red) changing color in response to the acid production.b. Sorbitol Nonfermenters: Escherichia coli serotype O157:H7 is a sorbitol nonfermenter. It ferments lactose but does not efficiently ferment sorbitol. Consequently, E. coli O157:H7 colonies on SMAC appear colorless or pale because they do not produce enough acid to change the indicator’s color.
- Additional Selectivity: Some modifications of SMAC include the addition of cefixime and tellurite. These compounds further inhibit the growth of non-O157 E. coli strains and unrelated bacteria like Proteus mirabilis, making SMAC more selective for E. coli O157:H7.
Clinical Applications of SMAC Agar:
Sorbitol MacConkey Agar (SMAC) is a specialized microbiological medium with several clinical applications, primarily focused on the detection and identification of pathogenic Escherichia coli serotype O157:H7. Here are the clinical applications of SMAC:
- Diagnosis of E. coli O157:H7 Infections: SMAC is primarily used in clinical laboratories to diagnose infections caused by Escherichia coli serotype O157:H7. This bacterium is associated with severe gastrointestinal illnesses, including hemorrhagic colitis and hemolytic uremic syndrome (HUS).
- Selective Isolation: SMAC is selective for E. coli O157:H7 because this strain is a sorbitol nonfermenter. While other E. coli strains ferment sorbitol, E. coli O157:H7 ferments sorbitol slowly or not at all. This selective property allows for the isolation of E. coli O157:H7 from clinical samples.
- Differential Identification: SMAC is a differential medium that differentiates between sorbitol-fermenting and sorbitol-nonfermenting bacteria. On SMAC, sorbitol-fermenting E. coli strains produce acid and appear as pink or red colonies due to the pH indicator’s color change. E. coli O157:H7, being a sorbitol nonfermenter, appears colorless or pale.
- Outbreak Investigations: In cases of suspected outbreaks of E. coli O157:H7 infections, SMAC is a valuable tool for confirming the presence of this pathogen. Rapid diagnosis is essential for effective outbreak control and management.
- Patient Management: Identifying E. coli O157:H7 in clinical samples helps healthcare providers initiate appropriate treatment and supportive care for infected patients. Early diagnosis can improve patient outcomes.
- Public Health Surveillance: SMAC is instrumental in monitoring the prevalence and distribution of E. coli O157:H7 infections at the population level. It aids in tracking the source of outbreaks and implementing preventive measures.
- Epidemiological Studies: The use of SMAC in clinical research and epidemiological studies contributes to a better understanding of the epidemiology and transmission dynamics of E. coli O157:H7.
- Foodborne Illness Investigations: When E. coli O157:H7 is suspected to be the cause of a foodborne illness outbreak, SMAC is used to detect and confirm its presence in food samples, facilitating the removal of contaminated products from the market.
Ingredients, Materials and composition of SMAC :
The composition of Sorbitol MacConkey Agar (SMAC) includes specific ingredients and materials designed to serve its selective and differential purposes, primarily the isolation and identification of Escherichia coli serotype O157:H7. Here is a general overview of the ingredients and materials used in SMAC:
Ingredients:
The exact Ingradients for SMAC may vary slightly based on specific formulations used by different manufacturers or laboratories, especially in terms of the precise concentrations of each ingredient.
- Pancreatic Digest of Gelatin: This provides a source of nitrogen and amino acids to support bacterial growth.
- Peptone: Another nitrogen source that aids bacterial growth and metabolic activity.
- Lactose: Lactose is included as a carbohydrate source. Bacteria capable of fermenting lactose produce acid, which is important for differentiation.
- Sorbitol: The key ingredient that distinguishes SMAC from regular MacConkey Agar. Sorbitol is a sugar alcohol used to identify sorbitol-nonfermenting strains of bacteria.
- Bile Salts: Bile salts inhibit the growth of gram-positive bacteria, which allows for the selective growth of gram-negative bacteria.
- Neutral Red: This pH indicator changes color based on the acidity of the medium. It turns red at a neutral pH and becomes colorless in an acidic environment.
- Crystal Violet: Crystal violet is an additional selective agent that inhibits the growth of gram-positive bacteria.
- Cefixime: In some variations of SMAC, cefixime, a cephalosporin antibiotic, may be added to further inhibit the growth of non-O157 E. coli strains.
- Potassium Tellurite: Tellurite is also used as a selective agent, particularly to inhibit the growth of Proteus mirabilis.
Materials:
- Agar: Agar is used as a solidifying agent, turning the medium into a gel-like substance that provides a surface for bacterial growth.
- Petri Dishes: Sterile Petri dishes are used to pour and solidify the agar medium.
- Autoclave: To sterilize the agar and other components before use.
- pH Meter: To ensure the medium’s pH falls within the appropriate range.
- Distilled Water: Used to prepare the agar medium.
- Bunsen Burner or Incubator: Depending on whether you’re preparing the medium or incubating bacterial cultures.
Composition of Sorbitol MacConkey Agar:
The exact composition of SMAC may vary slightly based on specific formulations used by different manufacturers or laboratories, especially in terms of the precise concentrations of each ingredient.
Here is a table outlining the typical composition of Sorbitol MacConkey Agar (SMAC), including the quantity and purpose of each component:
| Component | Quantity per Liter of Medium | Purpose |
|---|---|---|
| Pancreatic Digest of Gelatin | 20 grams | Provides nitrogen and amino acids for growth |
| Peptone | 5 grams | Aids in bacterial growth and metabolism |
| Lactose | 10 grams | Acts as a carbohydrate source |
| Sorbitol | 10 grams | Used to differentiate sorbitol nonfermenters |
| Bile Salts | 1.5 grams | Inhibits the growth of gram-positives |
| Neutral Red | 0.03 grams | pH indicator for differentiation |
| Crystal Violet | 0.001 grams | Additional selective agent against gram-positives |
| Cefixime (optional) | Variable | Further inhibits non-O157 E. coli strains (if added) |
| Potassium Tellurite (optional) | Variable | Selective agent, especially against Proteus mirabilis (if added) |
| Agar | 15-20 grams (varies) | Solidifying agent |
| Distilled Water | To make up to 1 liter | Medium solvent |
Preparation of Sorbitol MacConkey Agar:
- Weigh and Measure: Weigh each component accurately according to the specified quantities in the composition table (adjust for the desired batch size).
- Mix the Components: In a large container, add the weighed Pancreatic Digest of Gelatin, Peptone, Lactose, Sorbitol, Bile Salts, Neutral Red, and Crystal Violet to distilled water. Stir the mixture thoroughly to dissolve the ingredients.
- Adjust pH: Use a pH meter to check the pH of the mixture. The pH should be adjusted to around 7.1 to 7.3. If necessary, you can use hydrochloric acid (HCl) or sodium hydroxide (NaOH) to make pH adjustments. Ensure that the pH is within the acceptable range before proceeding.
- Add Agar: Sprinkle the Agar into the mixture while stirring continuously. Agar should be added gradually to prevent clumping. Stir until the agar is completely dissolved.
- Heat and Sterilize: Autoclave the mixture at 121°C for 15 minutes to sterilize it. Ensure that the agar is fully dissolved and the mixture is homogeneous before autoclaving.
- Cooling: Allow the medium to cool to around 45-50°C but not below its solidification point.
- Pour into Petri Dishes: In a sterile environment (e.g., laminar flow hood), pour the sterile SMAC agar into sterile Petri dishes to create agar plates. Fill each dish to an appropriate depth, usually around 15-20 mm.
- Solidification: Let the agar plates cool and solidify at room temperature. Avoid exposing them to drafts that can lead to uneven solidification.
- Label and Store: Label each Petri dish with the date, medium name, and any additional information required. Store the plates in a refrigerator at 2-8°C until needed.
Required Specimins for Culturing on SMAC Agar:
Sorbitol MacConkey Agar (SMAC) is primarily used for the selective isolation and identification of Escherichia coli serotype O157:H7, a pathogenic strain of E. coli. To culture specimens on SMAC agar, you typically require samples or specimens that may potentially contain this specific pathogen. Here are the types of specimens that are commonly cultured on SMAC agar:
- Stool Samples: Stool specimens are the most common samples cultured on SMAC agar for the detection of E. coli O157:H7. This bacterium is known to cause gastrointestinal infections, and its presence in stool can be indicative of infection.
- Food Samples: In cases of suspected foodborne outbreaks or contamination, food samples can be cultured on SMAC agar to check for the presence of E. coli O157:H7. This is particularly important for foods such as ground beef, leafy greens, and dairy products, which have been associated with outbreaks.
- Clinical Samples: Clinical samples other than stool, such as rectal swabs, anal swabs, or colonic mucosal biopsies, may be cultured on SMAC agar when gastrointestinal infection with E. coli O157:H7 is suspected.
- Environmental Samples: In cases where the source of an outbreak is unknown, environmental samples from food processing facilities, farms, or water sources can be cultured on SMAC agar to identify potential reservoirs of E. coli O157:H7.
Usage Procedure of Sorbitol MacConkey Agar:
The usage procedure of Sorbitol MacConkey Agar (SMAC) involves several steps to isolate and differentiate Escherichia coli serotype O157:H7 from other bacteria. Here is a step-by-step guide:
Materials and Equipment Needed:
- Sterile Sorbitol MacConkey Agar plates
- Specimens (e.g., stool samples, food samples, clinical samples)
- Inoculation loop or swab
- Incubator set to 35-37°C
- Biohazard waste disposal system
- Appropriate personal protective equipment (PPE)
Procedure:
- Prepare the Specimen: Ensure that the specimen you’re working with is properly collected and stored according to standard procedures. Follow appropriate biosafety protocols to handle potentially infectious samples.
- Label the Plates: Label the SMAC agar plates with the necessary information, including the sample source, date, and any other relevant identifiers.
- Inoculation: Using a sterile inoculation loop or swab, transfer a small amount of the specimen onto the surface of the SMAC agar plate. Streak the sample in a zigzag or streak pattern to distribute it evenly over the surface. Be careful not to puncture the agar.
- Incubation: Place the inoculated SMAC plates into an incubator set at 35-37°C. Incubate the plates for 24-48 hours. E. coli O157:H7 typically grows on SMAC agar within this time frame.
- Observation: After incubation, examine the plates for bacterial growth and colony characteristics. Pay attention to the following:
- Pink or red colonies: Most E. coli strains will ferment sorbitol and produce acid, resulting in pink or red colonies.
- Colorless or pale colonies: Escherichia coli serotype O157:H7 does not efficiently ferment sorbitol, so it typically produces colorless or pale colonies on SMAC agar.
- Confirmation: To confirm the presence of E. coli O157:H7, further biochemical tests or molecular techniques may be necessary. Positive colonies should be subjected to additional testing, such as latex agglutination or PCR, for confirmation.
- Record Results: Document the results, including the number and characteristics of colonies, and any confirmatory test results, in your laboratory records.
- Safety Precautions: Follow appropriate biosafety practices throughout the procedure, and dispose of specimens and materials properly in a biohazard waste disposal system.
- Data Interpretation: Interpret the results based on the colony appearance and confirmatory tests. Positive results may indicate the presence of E. coli O157:H7, which can have implications for patient care or food safety measures.
Result Interpretation of Sorbitol MacConkey Agar:
1. Colony Appearance:
- Pink or Red Colonies: These colonies indicate the presence of E. coli strains that ferment sorbitol efficiently. Most non-pathogenic E. coli strains will produce pink or red colonies due to the production of acid during sorbitol fermentation.
- Colorless or Pale Colonies: These colonies are typically indicative of Escherichia coli serotype O157:H7 or other sorbitol-nonfermenting E. coli strains. These bacteria do not efficiently ferment sorbitol, so they do not produce sufficient acid to change the pH indicator’s color, resulting in colorless or pale colonies.
2. Interpretation:
- Positive Result: Colorless or pale colonies are considered a positive result for potential E. coli O157:H7. It suggests that the specimen may contain E. coli O157:H7 or another sorbitol-nonfermenting E. coli strain.
- Negative Result: Pink or red colonies are considered a negative result for E. coli O157:H7. In this case, it suggests that the specimen likely does not contain E. coli O157:H7.
3. Confirmation:
- While SMAC agar is a useful screening tool, further confirmation is usually required to definitively identify E. coli O157:H7. Confirmatory tests may include:
- Latex agglutination assays to detect O157 antigens.
- Polymerase Chain Reaction (PCR) assays targeting specific genetic markers associated with E. coli O157:H7.
- Biochemical tests to confirm other characteristics of E. coli O157:H7, such as its inability to ferment sorbitol.
Growth of Bacterias on Sorbitol MacConkey Agar:
1. Escherichia coli O157:H7:
- Growth: Colorless or pale colonies.
- Sorbitol Fermentation: Slow or absent (sorbitol nonfermenter).
- Interpretation: Positive for E. coli O157:H7.
2. Non-O157 Escherichia coli Strains:
- Growth: Pink or red colonies.
- Sorbitol Fermentation: Efficient sorbitol fermenters.
- Interpretation: Negative for E. coli O157:H7.
3. Non-pathogenic E. coli Strains:
- Growth: Pink or red colonies.
- Sorbitol Fermentation: Efficient sorbitol fermenters.
- Interpretation: Negative for E. coli O157:H7.
4. Other Enteric Bacteria:
- Growth: Variable, depending on the species.
- Sorbitol Fermentation: May or may not ferment sorbitol.
- Interpretation: Typically negative for E. coli O157:H7.
5. Proteus mirabilis:
- Growth: Variable, but usually grows.
- Sorbitol Fermentation: Negative (sorbitol nonfermenter).
- Interpretation: Negative for E. coli O157:H7. The selective agents may inhibit growth in some cases.
6. Other Sorbitol-Nonfermenting Bacteria:
- Growth: Variable, depending on the species.
- Sorbitol Fermentation: Negative (sorbitol nonfermenter).
- Interpretation: Typically negative for E. coli O157:H7.
Coloney Characteristics on SMAC Agar:
| Bacterial Strain | Growth Appearance | Sorbitol Fermentation | Interpretation |
|---|---|---|---|
| Escherichia coli O157:H7 | Colorless or Pale Colonies | Slow or Absent | Positive for E. coli O157:H7 |
| Non-O157 Escherichia coli Strains | Pink or Red Colonies | Efficient Fermenters | Negative for E. coli O157:H7 |
| Non-Pathogenic E. coli Strains | Pink or Red Colonies | Efficient Fermenters | Negative for E. coli O157:H7 |
| Other Enteric Bacteria | Variable Growth | Variable Sorbitol Fermentation | Typically Negative for E. coli O157:H7 |
| Proteus mirabilis | Variable Growth | Negative (Sorbitol Nonfermenter) | Negative for E. coli O157:H7 |
| Other Sorbitol-Nonfermenting Bacteria | Variable Growth | Negative (Sorbitol Nonfermenter) | Typically Negative for E. coli O157:H7 |
Limitations of Sorbitol MacConkey Agar:
- Specificity for E. coli O157:H7: SMAC is designed specifically to identify E. coli O157:H7 based on its inability to efficiently ferment sorbitol. It may not detect other pathogenic E. coli strains or non-O157 serotypes, which can also cause illnesses.
- False Negatives: While SMAC is a reliable indicator for E. coli O157:H7, there can be false negatives. Some strains of E. coli O157:H7 may exhibit variable sorbitol fermentation, leading to inconclusive results.
- Lack of Identification: SMAC does not identify bacterial species beyond the differentiation of sorbitol-fermenting and sorbitol-nonfermenting strains. Further biochemical or molecular testing is required to confirm the identity of specific strains.
- Inhibitory Effects: The selective agents in SMAC may inhibit the growth of some bacterial species, potentially leading to false-negative results for those bacteria or reduced recovery rates in mixed cultures.
- Limited Use for Non-O157 E. coli: SMAC is primarily designed for detecting E. coli O157:H7. It may not be suitable for the isolation of other non-O157 pathogenic E. coli strains or other enteric pathogens.
- Time-Consuming: The incubation period for SMAC is typically 24-48 hours, which may be considered a relatively long time for clinical or food safety applications, especially in urgent cases.
- Cost and Availability: Some laboratories may find the cost of SMAC agar and the availability of commercial media to be limiting factors, particularly in resource-limited settings.
- Other Selective Media: In certain situations, alternative selective media may be preferred over SMAC for detecting E. coli O157:H7, depending on the specific requirements of the laboratory or the nature of the samples.
- Requires Confirmatory Tests: SMAC alone is not sufficient for the final identification of E. coli O157:H7. Confirmatory tests, such as PCR or immunological assays, are typically needed to confirm the presence of this pathogen.
- Sample Preparation: Proper collection and handling of specimens are crucial. Inadequate sample collection, transport, or storage conditions can affect the sensitivity and accuracy of SMAC testing.
Safety Considerations of Sorbitol MacConkey Agar:
Safety is paramount when working with microbiological media like Sorbitol MacConkey Agar (SMAC), especially since it may involve potentially pathogenic bacteria. Here are some safety considerations to keep in mind when handling SMAC:
- Personal Protective Equipment (PPE):
- Always wear appropriate PPE, including lab coats, gloves, safety goggles, and, in some cases, face shields.
- Ensure that PPE is in good condition and properly fitted.
- Biosafety Cabinet (BSC):
- When working with potentially infectious specimens or cultures, use a Class II BSC or laminar flow hood to minimize the risk of exposure to aerosols.
- Perform procedures carefully within the BSC to prevent contamination of the workspace and protect yourself.
- Good Laboratory Practices:
- Adhere to good laboratory practices (GLP) and aseptic techniques to minimize the risk of contamination.
- Avoid touching your face, mouth, or eyes while working in the laboratory.
- Handling of Specimens:
- Treat all clinical or environmental specimens as potentially infectious.
- Follow institutional and regulatory guidelines for proper collection, transport, and disposal of specimens.
- Sterilization and Autoclaving:
- Ensure that all equipment and materials that come into contact with SMAC are properly sterilized or autoclaved before and after use.
- Follow autoclave safety procedures to prevent burns and exposure to hot materials.
- Labeling and Documentation:
- Properly label SMAC plates with information such as the specimen source, date, and any other relevant identifiers.
- Maintain accurate records of procedures and results for traceability.
- Waste Disposal:
- Dispose of used SMAC plates and potentially contaminated materials in accordance with biohazard waste disposal guidelines.
- Follow local, regional, and national regulations for hazardous waste disposal.
- Incubation and Storage:
- Incubate SMAC plates in a designated incubator set to the appropriate temperature (typically 35-37°C).
- Store SMAC plates in a secure location within the incubator, away from potentially hazardous materials.
- Emergency Procedures:
- Know the location of safety showers, eyewash stations, and fire extinguishers in the laboratory.
- Familiarize yourself with laboratory-specific emergency protocols, including what to do in case of spills, fires, or accidents.
- Training and Education:
- Ensure that laboratory personnel are properly trained in microbiological techniques and safety procedures.
- Regularly review safety protocols and provide ongoing education and training.
- Risk Assessment:
- Conduct a risk assessment before performing experiments involving potentially hazardous microorganisms to identify and mitigate risks.
- Reporting and Incident Response:
- Report any accidents, incidents, or potential exposures to laboratory management and follow institutional protocols for incident response.
Comparison of SMAC Agar with Other Microbiological Media:
| Property/Characteristic | Sorbitol MacConkey Agar (SMAC) | MacConkey Agar | Eosin Methylene Blue (EMB) Agar | Blood Agar |
|---|---|---|---|---|
| Primary Purpose | Identification of E. coli O157:H7 | Differentiation of lactose fermenters and non-fermenters | Differentiation of lactose fermenters and non-fermenters | Hemolysis pattern of bacterial isolates |
| Selective Agents | Bile salts, crystal violet, cefixime, potassium tellurite | Bile salts, crystal violet | Eosin, methylene blue | None (non-selective) |
| Differential Characteristics | Sorbitol fermentation (colorless or pink colonies) | Lactose fermentation (pink colonies) | Lactose fermentation (dark colonies) | Hemolysis (alpha, beta, gamma) |
| Target Organisms | E. coli O157:H7 and sorbitol-nonfermenting E. coli strains | Various enteric bacteria | Various enteric bacteria | Various bacteria, particularly those with hemolytic properties |
| Application | Specific detection of E. coli O157:H7, particularly in clinical and food safety settings | General differentiation of lactose-fermenting and non-fermenting enteric bacteria | General differentiation of lactose-fermenting and non-fermenting enteric bacteria | Assessment of hemolytic activity in bacteria |
| Additional Considerations | May include cefixime and tellurite for further selectivity | Useful for Enterobacteriaceae identification | EMB agar may inhibit some non-lactose fermenting organisms | Blood agar may contain sheep’s blood for hemolysis testing |
| Limitations | Limited to detecting E. coli O157:H7 and related sorbitol-nonfermenting strains | May not be selective enough for specific bacterial identification | EMB agar may inhibit some bacteria and select against certain non-lactose fermenters | Not selective for specific bacteria, but useful for general bacterial growth |
| Use in Microbiology | Commonly used in clinical microbiology and food safety laboratories | Commonly used for general enteric bacterial differentiation | Commonly used for general enteric bacterial differentiation | Commonly used for bacterial isolation and hemolysis testing |
Future Trends in E. Coli Serotypes Detection:
The detection and characterization of Escherichia coli (E. coli) serotypes, including pathogenic strains like E. coli O157:H7, continue to evolve with advancements in technology and scientific research. Future trends in E. coli serotype detection are likely to be shaped by the following developments:
- Molecular Techniques:
- Whole Genome Sequencing (WGS): WGS provides comprehensive genetic information about bacterial isolates, allowing for precise identification of serotypes and subtypes. It can also reveal virulence factors and antimicrobial resistance genes.
- PCR-Based Assays: PCR assays targeting specific genes or markers associated with E. coli serotypes will continue to be valuable for rapid and accurate detection.
- High-Throughput Technologies:
- Microarrays and Next-Generation Sequencing (NGS): These technologies can analyze multiple isolates simultaneously, facilitating large-scale surveillance and outbreak investigations.
- Bioinformatics and Data Analysis:
- Advanced data analysis techniques will enable the identification of unique genetic markers and patterns associated with different E. coli serotypes, enhancing our understanding of their diversity and evolution.
- Point-of-Care Diagnostics:
- The development of portable and rapid diagnostic devices will allow for on-site detection of E. coli serotypes in clinical and field settings, enabling quicker responses to outbreaks.
- Biosensors:
- Biosensor technologies are being explored for their ability to detect specific E. coli serotypes quickly and with high sensitivity. These devices can be integrated into food safety and clinical testing.
- Machine Learning and AI:
- Machine learning algorithms can analyze vast datasets and predict the presence of specific serotypes based on genetic patterns, aiding in early detection and surveillance.
- Bacterial Strain Typing:
- Advancements in typing methods, such as Multi-Locus Sequence Typing (MLST) and Single Nucleotide Polymorphism (SNP) analysis, will help differentiate closely related serotypes.
- Antibiotic Resistance Surveillance:
- Integration of serotype detection with antimicrobial resistance profiling will become more critical as antibiotic-resistant E. coli strains continue to emerge.
- Rapid and Multiplex Testing:
- Development of multiplex assays that simultaneously detect multiple serotypes, virulence factors, and resistance genes will improve efficiency in clinical and food safety laboratories.
- Public Health Genomics:
- Genomic epidemiology will play a central role in understanding the transmission dynamics of E. coli serotypes, assisting in outbreak investigations and public health interventions.
- Global Collaboration:
- Enhanced global collaboration and data sharing among public health agencies, researchers, and laboratories will facilitate the monitoring and control of E. coli serotypes on a broader scale.
- Environmental Surveillance:
- Increased surveillance of environmental sources, such as water and wildlife, will contribute to a better understanding of the reservoirs and transmission pathways of E. coli serotypes.
FAQs:
1. What is Sorbitol MacConkey Agar (SMAC)?
- Sorbitol MacConkey Agar is a specialized microbiological growth medium used for the selective isolation and differentiation of Escherichia coli serotype O157:H7 and other sorbitol-nonfermenting E. coli strains.
2. Why is SMAC used in microbiology?
- SMAC is used to identify E. coli O157:H7, a pathogenic strain responsible for foodborne illnesses. It differentiates this strain from other E. coli based on its sorbitol fermentation characteristics.
3. How does SMAC differentiate E. coli O157:H7 from other E. coli strains?
- E. coli O157:H7 typically ferments sorbitol slowly or not at all, leading to colorless or pale colonies on SMAC agar, while most other E. coli strains ferment sorbitol and produce pink or red colonies.
4. What are the components of SMAC agar?
- SMAC contains ingredients like pancreatic digest of gelatin, peptone, lactose, sorbitol, bile salts, neutral red, crystal violet, and agar. Additional selective agents such as cefixime and tellurite may be included.
5. What is the purpose of cefixime and tellurite in SMAC agar?
- Cefixime and tellurite are added to SMAC to inhibit the growth of non-O157 E. coli strains, Proteus mirabilis, and other sorbitol-nonfermenting strains that may interfere with the detection of E. coli O157:H7.
6. What specimens are cultured on SMAC agar?
- Stool samples, food samples, clinical samples, and environmental samples suspected of containing E. coli O157:H7 or sorbitol-nonfermenting E. coli strains can be cultured on SMAC agar.
7. What do the different colony colors on SMAC agar indicate?
- Colorless or pale colonies indicate potential E. coli O157:H7, while pink or red colonies suggest the presence of other E. coli strains that efficiently ferment sorbitol.
8. Is SMAC agar a definitive test for E. coli O157:H7?
- No, SMAC agar is a presumptive test. Confirmatory tests, such as PCR or immunological assays, are typically required to definitively identify E. coli O157:H7.
9. What are the limitations of SMAC agar?
- SMAC is selective for E. coli O157:H7 and sorbitol-nonfermenting strains but may not detect other pathogenic E. coli serotypes. Some strains may exhibit variable sorbitol fermentation, leading to inconclusive results.
10. How should SMAC agar be stored and handled?
- Store SMAC agar plates in a refrigerator at 2-8°C before use. Handle them aseptically to prevent contamination, and follow appropriate biosafety protocols when working with potential pathogens.
Conclusion:
In conclusion, Sorbitol MacConkey Agar (SMAC) is a valuable tool in microbiology and clinical laboratories, primarily used for the selective isolation and differentiation of Escherichia coli serotype O157:H7 and other sorbitol-nonfermenting E. coli strains. Its unique ability to differentiate these pathogenic strains from other E. coli is crucial in clinical diagnostics and food safety.
SMAC agar contains selective agents like bile salts, crystal violet, cefixime, and tellurite, which inhibit the growth of non-target bacteria, allowing for the isolation of the specific strains of interest. Its interpretation is based on the color of bacterial colonies, with colorless or pale colonies indicating potential E. coli O157:H7.
However, SMAC has limitations, including the need for confirmatory tests to definitively identify E. coli O157:H7 and its selectivity for specific serotypes. Future trends in E. coli serotype detection are likely to involve molecular techniques, high-throughput technologies, and advanced data analysis, leading to more rapid and precise identification of pathogenic strains.
Possible References Used




One Comment