Microbiology Testing SOPs are important documents that provide step-by-step instructions for performing various laboratory procedures, including microbiology testing. These procedures are critical to ensure the accuracy, precision, and reproducibility of test results, as well as to maintain a safe laboratory environment.

23 Guidelines for Creating Effective Microbiology Testing SOPs
Below are some general guidelines for creating microbiology testing SOPs:
- Purpose: Clearly define the purpose of the SOP, including the test(s) being performed, the expected outcome, and any potential risks associated with the procedure.
- Identify the test: Clearly identify the test that needs to be performed, including the purpose of the test, the sample type, and any specific testing requirements.
- Sample collection: Clearly outline the procedures for sample collection, including the type of sample, sample volume, and the proper technique for collecting the sample.
- Sample storage and transport: Clearly define the temperature, storage time, and any specific requirements for sample storage and transport to the laboratory to ensure the integrity of the sample.
- Sample preparation: Define the specific protocols for sample preparation, including any required reagents, equipment, or steps that need to be taken to ensure proper sample homogenization.
- Sterilization procedures: Define the specific procedures for sterilizing equipment, media, and surfaces to avoid any contamination during testing.
- Media preparation: Clearly outline the procedure for media preparation, including the type of media, the required ingredients, and the sterilization method.
- Testing procedures: Describe step-by-step instructions for conducting the microbiology test. Include information on the specific media to use, the incubation temperature and duration, and any other pertinent information.
- Inoculation of samples: The SOP should outline the procedures for properly handling and inoculating samples onto the appropriate media. This may include instructions for diluting samples, using sterile technique, and ensuring that the appropriate amount of sample is added to the media.
- Incubation: After inoculating the samples, the media should be incubated under the appropriate conditions (temperature, humidity, and time) to allow for microbial growth. The SOP should specify the incubation parameters for each type of test.
- Microbial identification and Interpretation: Define the protocol for microbial identification, including the use of appropriate biochemical tests, DNA sequencing, or other methods.
- Microscopic examination: This section should describe the procedures for preparing and examining samples under a microscope, including staining methods and interpretation of results.
- Biochemical testing: This section should detail the procedures for conducting biochemical tests to identify specific microorganisms, including the selection of appropriate tests, preparation of reagents, and interpretation of results.
- Quality control: Define the quality control procedures for ensuring accurate and reliable results, including the use of appropriate positive and negative controls.
- Troubleshooting: Include troubleshooting tips for common problems that may arise during the test, including any potential sources of error and their solutions.
- Equipment Maintenance SOP: Regular maintenance of equipment used in microbiology testing is critical to ensure accurate results. This SOP should detail how to maintain equipment, when to perform maintenance, and what to do in case of equipment failure.
- Safety SOP: Working in a microbiology laboratory can be dangerous, so safety procedures are essential to prevent accidents or exposure to harmful microorganisms. This SOP should include details on how to handle hazardous materials, how to dispose of waste, and what to do in case of an emergency.
- Data recording and analysis: Define the procedure for recording and analyzing data, including the use of appropriate software, databases, and statistical analysis methods.
- Reporting of results: Clearly define the procedure for reporting results, including the format, units, and any reference ranges used.
- Review and approval: Before implementing the SOP, it should be reviewed by appropriate personnel, such as a laboratory manager, to ensure that it is accurate, complete, and compliant with relevant regulations and standards.
- Disposal of samples and materials: Define the procedure for the proper disposal of samples, equipment, and materials used during the testing process, including any hazardous or biohazardous waste.
- Training and implementation: Once the SOP has been approved, it should be implemented and all personnel involved in testing should receive appropriate training on the procedures outlined in the SOP.
- Record keeping: Finally, it is important to keep detailed records of all testing procedures, including sample identification, media used, incubation parameters, and results. The SOP should include guidelines for record keeping to ensure that all necessary information is recorded accurately and consistently.
By following these SOPs, microbiology testing laboratories can ensure consistent and accurate results, which are essential for clinical diagnosis, research, and other applications.
Purpose of SOPs:
The purpose of Standard Operating Procedures (SOPs) for microbiology testing is to provide a standardized approach to performing specific microbiology tests in a laboratory. The use of SOPs is critical to ensure the accuracy, precision, and reproducibility of test results, as well as to maintain a safe laboratory environment.
Microbiology testing SOPs serve several key purposes, including:
- Ensuring consistency: SOPs provide a standardized approach to performing microbiology tests, ensuring that they are performed consistently and reliably every time.
- Maintaining quality: By providing clear and detailed instructions, SOPs can help to maintain the quality of microbiology testing, ensuring that test results are accurate and reliable.
- Improving efficiency: SOPs can help to streamline processes and reduce errors, improving the efficiency of microbiology testing.
- Meeting regulatory requirements: SOPs are often required by regulatory agencies, such as the Clinical and Laboratory Standards Institute (CLSI) and the U.S. Food and Drug Administration (FDA), to ensure compliance with relevant regulations and guidelines.
- Facilitating training: SOPs can be used as a tool to train new laboratory personnel on how to perform microbiology tests correctly and consistently.
Overall, microbiology testing SOPs are an essential component of laboratory quality management systems and are critical to ensuring the accuracy and reliability of microbiology test results.
Identify the test SOP:
Clearly identify the test that needs to be performed, including the purpose of the test, the sample type, and any specific testing requirements.
There are many different types of microbiology tests, and each one requires its own specific SOP. Here are some examples of common microbiology tests and the associated SOPs:
- Gram staining: This test is used to identify bacteria based on their cell wall structure. The SOP for Gram staining would include the steps for preparing the bacterial sample, staining the sample, and interpreting the results.
- Culture and sensitivity testing: This test is used to determine the type of bacteria present in a sample and to identify which antibiotics are effective against the bacteria. The SOP for culture and sensitivity testing would include the steps for preparing the bacterial sample, inoculating the sample onto various culture media, incubating the cultures, and interpreting the results.
- Polymerase Chain Reaction (PCR) testing: This test is used to amplify and detect DNA or RNA from a pathogen. The SOP for PCR testing would include the steps for preparing the sample, setting up the PCR reaction, running the PCR reaction, and interpreting the results.
- Antibiotic susceptibility testing: This test is used to determine which antibiotics are effective against a specific strain of bacteria. The SOP for antibiotic susceptibility testing would include the steps for preparing the bacterial sample, inoculating the sample onto various antibiotic-containing media, incubating the cultures, and interpreting the results.
- ELISA testing: This test is used to detect the presence of antibodies or antigens in a sample. The SOP for ELISA testing would include the steps for preparing the sample, setting up the ELISA assay, running the assay, and interpreting the results.
Overall, the SOP for each microbiology test will vary depending on the specific test being performed and the laboratory’s protocols and procedures.
Sample collection SOP:
The sample collection SOP is an essential component of microbiology testing as it ensures the integrity and quality of the sample being tested. Here are some general steps that may be included in a sample collection SOP for microbiology testing:
- Identify the sample source: The SOP should specify the type of sample to be collected and the location from which it will be collected.
- Select appropriate collection materials: The SOP should provide guidance on the type of collection materials to be used, such as swabs, sterile containers, or transport media.
- Ensure aseptic technique: The SOP should outline the procedures to be followed to ensure that the sample is collected using aseptic technique to avoid contamination. This may include hand hygiene, wearing appropriate personal protective equipment (PPE), and sterilizing collection materials.
- Collect the sample: The SOP should provide step-by-step instructions on how to collect the sample, including the type and amount of sample to be collected, the method of collection, and any specific handling requirements.
- Label the sample: The SOP should specify how the sample should be labeled to ensure accurate identification and traceability. This may include information such as patient name, date and time of collection, and any relevant clinical information.
- Transport the sample: The SOP should provide guidance on how to transport the sample to the laboratory, including any specific temperature or time requirements.
Overall, a sample collection SOP is critical to ensuring that the sample collected is of high quality and suitable for microbiology testing. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the test results are accurate and reliable.
Sample storage and transport SOP:
The sample storage and transport SOP is an important part of microbiology testing, as it helps to ensure that the sample remains stable and suitable for analysis during transport and storage. Here are some general steps that may be included in a sample storage and transport SOP for microbiology testing:
- Determine the appropriate storage conditions: The SOP should specify the temperature and any other storage conditions required for the specific type of sample being tested. This may include requirements for refrigeration, freezing, or use of a specific transport medium.
- Label the sample: The SOP should provide instructions on how to label the sample appropriately, including patient information, sample type, and any other relevant information.
- Package the sample for transport: The SOP should specify how the sample should be packaged to ensure its integrity during transport. This may include the use of insulated containers, gel packs, or other materials to maintain the appropriate temperature.
- Document the transport conditions: The SOP should specify how the transport conditions should be documented, including the temperature of the sample during transport and any deviations from the recommended storage conditions.
- Deliver the sample to the laboratory: The SOP should provide instructions on how to deliver the sample to the laboratory, including any specific delivery requirements and the timeframe within which the sample should be delivered.
- Store the sample: The SOP should provide guidance on how to store the sample upon receipt at the laboratory, including any specific storage requirements.
Overall, a sample storage and transport SOP is critical to ensuring that the sample remains stable and suitable for microbiology testing during transport and storage. The SOP should be followed closely to minimize the risk of sample degradation or contamination and to ensure that the test results are accurate and reliable.
Sample Preparation SOP:
The sample preparation SOP is a crucial component of microbiology testing as it ensures that the sample is properly prepared for analysis. Here are some general steps that may be included in a sample preparation SOP for microbiology testing:
- Prepare the sample for processing: The SOP should specify the methods and materials to be used to prepare the sample for analysis. This may include filtration, centrifugation, or other techniques.
- Inoculate the appropriate culture media: The SOP should provide instructions on how to inoculate the appropriate culture media with the prepared sample. This may involve streaking or spreading the sample on the culture media or inoculating the sample into liquid media.
- Incubate the cultures: The SOP should provide guidance on how to incubate the cultures, including the temperature and duration of incubation, as well as any specific atmospheric conditions required for the growth of the microorganisms.
- Perform colony identification: The SOP should specify the methods for colony identification, including visual inspection of the cultures, gram staining, and other biochemical tests as needed.
- Perform sensitivity testing: The SOP should provide instructions on how to perform sensitivity testing to determine the susceptibility of the microorganisms to various antibiotics or other treatments.
- Record and report the results: The SOP should specify how the results should be recorded and reported, including any quality control measures that need to be taken.
Overall, a sample preparation SOP is critical to ensuring that the sample is properly processed and analyzed for microbiology testing. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the test results are accurate and reliable.
Sterilization Procedures SOP:
Sterilization procedures are an essential component of microbiology testing, as they help to prevent the introduction of contaminating microorganisms into the testing process. Here are some general steps that may be included in a sterilization procedures SOP for microbiology testing:
- Identify the materials to be sterilized: The SOP should specify the materials to be sterilized, such as laboratory equipment, media, and other supplies.
- Select the appropriate sterilization method: The SOP should provide guidance on the appropriate sterilization method to be used for the specific materials being sterilized. This may include autoclaving, dry heat sterilization, or chemical sterilization.
- Prepare the materials for sterilization: The SOP should provide instructions on how to prepare the materials for sterilization, including any cleaning or decontamination procedures required prior to sterilization.
- Sterilize the materials: The SOP should specify how to perform the sterilization process, including the appropriate settings, time, and temperature required for the specific sterilization method being used.
- Monitor the sterilization process: The SOP should provide guidance on how to monitor the sterilization process to ensure that it is effective. This may include using sterilization indicators, such as biological or chemical indicators, to verify that the sterilization process has been successful.
- Store the sterilized materials: The SOP should provide instructions on how to store the sterilized materials to maintain their sterility until they are ready to be used.
Overall, a sterilization procedures SOP is critical to ensuring that the materials used in microbiology testing are free from contamination and that the test results are accurate and reliable. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the sterilization process is effective.
Media Preparation SOP:
Media preparation is a critical component of microbiology testing, as it provides the nutrient-rich environment necessary for the growth of microorganisms. Here are some general steps that may be included in a media preparation SOP for microbiology testing:
- Select the appropriate media: The SOP should specify the appropriate media to be used for the specific microorganisms being tested. This may include agar plates, broth, or other types of media.
- Prepare the media: The SOP should provide guidance on how to prepare the media, including the materials needed and the appropriate measurements of each ingredient. The media should be prepared in a sterile environment to prevent contamination.
- Dispense the media: The SOP should provide instructions on how to dispense the media into appropriate containers, such as Petri dishes or test tubes. The containers should also be sterile to prevent contamination.
- Sterilize the media: The SOP should specify how to sterilize the media to ensure that it is free from contamination. This may involve autoclaving or other sterilization methods.
- Store the media: The SOP should provide instructions on how to store the media to maintain its quality and sterility until it is ready to be used.
- Quality control: The SOP should specify any quality control measures that need to be taken to ensure the media is performing as expected. This may include using positive and negative controls to verify that the media is able to support the growth of the microorganisms being tested.
Overall, a media preparation SOP is critical to ensuring that the media used in microbiology testing is of high quality and free from contamination. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the test results are accurate and reliable.
Testing Procedures SOP:
Testing procedures SOPs outline the specific steps that need to be followed when conducting microbiology testing. These SOPs are critical to ensuring that the testing is performed consistently and accurately. Here are some general steps that may be included in a testing procedures SOP for microbiology testing:
- Prepare the sample: The SOP should specify how to prepare the sample for testing, including any required dilutions or other preparations.
- Inoculate the media: The SOP should provide instructions on how to inoculate the media with the prepared sample, including the appropriate volume and technique to be used.
- Incubate the media: The SOP should specify the appropriate temperature, humidity, and time required for incubation of the media.
- Observe and record results: The SOP should specify how to observe and record the results of the testing, including any specific characteristics or criteria to look for.
- Perform confirmatory testing: The SOP should specify any confirmatory testing that needs to be performed to confirm the identity or characteristics of the microorganism being tested.
- Interpret results: The SOP should provide guidance on how to interpret the results of the testing, including any criteria for determining positive or negative results.
- Dispose of waste: The SOP should specify how to dispose of any waste generated during the testing process, including contaminated media or other materials.
Overall, a testing procedures SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the test results are accurate and reliable.
Inoculation of samples SOP:
Inoculation of samples is a critical step in microbiology testing, as it involves transferring a small amount of the sample onto a growth medium to allow microorganisms to grow and form colonies. Here are some general steps that may be included in an inoculation of samples SOP for microbiology testing:
- Prepare the inoculating tools: The SOP should specify how to prepare the inoculating tools, including any required sterilization procedures.
- Label the media: The SOP should specify how to label the media to ensure that it can be tracked and identified throughout the testing process.
- Transfer the sample: The SOP should provide instructions on how to transfer a small amount of the sample onto the media using the prepared inoculating tool.
- Incubate the media: The SOP should specify the appropriate temperature, humidity, and time required for incubation of the media.
- Observe and record results: The SOP should specify how to observe and record the results of the inoculation, including any specific characteristics or criteria to look for.
- Perform confirmatory testing: The SOP should specify any confirmatory testing that needs to be performed to confirm the identity or characteristics of the microorganism being tested.
- Dispose of waste: The SOP should specify how to dispose of any waste generated during the inoculation process, including contaminated media or other materials.
Overall, an inoculation of samples SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the test results are accurate and reliable.
Incubation SOP:
Incubation is a critical step in microbiology testing, as it involves providing the ideal environment for microorganisms to grow and form colonies. Here are some general steps that may be included in an incubation SOP for microbiology testing:
- Label the media: The SOP should specify how to label the media to ensure that it can be tracked and identified throughout the testing process.
- Inoculate the media: The SOP should specify how to inoculate the media with the prepared sample, including the appropriate volume and technique to be used.
- Incubate the media: The SOP should specify the appropriate temperature, humidity, and time required for incubation of the media, as well as any other specific conditions or requirements.
- Monitor the incubation: The SOP should specify how to monitor the incubation process to ensure that the conditions are maintained and any issues are identified and addressed promptly.
- Record results: The SOP should specify how to record the results of the incubation process, including any specific characteristics or criteria to look for.
- Perform confirmatory testing: The SOP should specify any confirmatory testing that needs to be performed to confirm the identity or characteristics of the microorganism being tested.
- Dispose of waste: The SOP should specify how to dispose of any waste generated during the incubation process, including contaminated media or other materials.
Overall, an incubation SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the test results are accurate and reliable.
Microbial identification and Interpretation SOP:
Microbial identification and interpretation is the final step in microbiology testing, where the results obtained from the testing procedures are used to identify and interpret the type of microorganism present in the sample. Here are some general steps that may be included in a microbial identification and interpretation SOP for microbiology testing:
- Collect all data: The SOP should specify how to collect all relevant data from the testing procedures, including colony characteristics, biochemical tests, and any other results obtained.
- Analyze the data: The SOP should specify how to analyze the data obtained, including any criteria or guidelines used to identify the microorganism present in the sample.
- Confirm the identification: The SOP should specify any confirmatory testing that needs to be performed to confirm the identification of the microorganism, such as using molecular techniques.
- Interpret the results: The SOP should specify how to interpret the results obtained from the testing procedures, including any guidelines or standards used to determine the significance of the findings.
- Record the results: The SOP should specify how to record the results of the microbial identification and interpretation process, including any specific characteristics or criteria used to identify the microorganism and the final interpretation of the results.
- Report the results: The SOP should specify how to report the results to the appropriate personnel, such as healthcare providers or public health officials, as needed.
Overall, a microbial identification and interpretation SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or misinterpretation of the results and to ensure that the final interpretation is accurate and reliable.
Microscopic examination SOP:
Microscopic examination is a key step in microbiology testing, where the sample is observed under a microscope to identify and characterize microorganisms. Here are some general steps that may be included in a microscopic examination SOP for microbiology testing:
- Prepare the microscope: The SOP should specify how to prepare the microscope for use, including any cleaning or calibration that needs to be performed.
- Prepare the sample: The SOP should specify how to prepare the sample for observation under the microscope, such as by fixing or staining the sample.
- Observe the sample: The SOP should specify how to observe the sample under the microscope, including the appropriate magnification and lighting conditions to use.
- Identify microorganisms: The SOP should specify how to identify microorganisms present in the sample based on their characteristics, such as size, shape, and staining properties.
- Record results: The SOP should specify how to record the results of the microscopic examination, including any specific characteristics or criteria used to identify the microorganisms observed.
- Perform confirmatory testing: The SOP should specify any confirmatory testing that needs to be performed to confirm the identity or characteristics of the microorganisms observed, such as using molecular techniques.
- Dispose of waste: The SOP should specify how to dispose of any waste generated during the microscopic examination process, including contaminated slides or other materials.
Overall, a microscopic examination SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the identification of microorganisms is accurate and reliable.
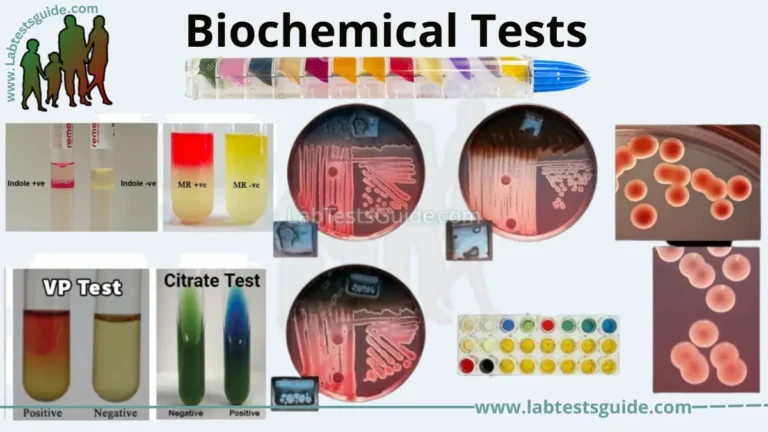
Biochemical testing SOP:
Biochemical testing is a key step in microbiology testing, where specific biochemical reactions are used to identify and characterize microorganisms. Here are some general steps that may be included in a biochemical testing SOP for microbiology testing:
- Prepare the test materials: The SOP should specify how to prepare the necessary materials for the biochemical tests, such as culture media and reagents.
- Inoculate the test samples: The SOP should specify how to inoculate the test samples into the appropriate culture media to promote growth and metabolic activity.
- Perform the biochemical tests: The SOP should specify how to perform the specific biochemical tests required to identify the microorganisms, including any specific reagents or methods used.
- Record the results: The SOP should specify how to record the results of the biochemical tests, including any criteria used to interpret the results.
- Confirm the identification: The SOP should specify any confirmatory testing that needs to be performed to confirm the identification of the microorganism, such as using molecular techniques.
- Dispose of waste: The SOP should specify how to dispose of any waste generated during the biochemical testing process, including contaminated materials and culture media.
Overall, a biochemical testing SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the identification of microorganisms is accurate and reliable.
Quality control SOP:
Quality control is a crucial aspect of microbiology testing, and a quality control SOP should be established to ensure that the results generated are accurate and reliable. Here are some general steps that may be included in a quality control SOP for microbiology testing:
- Establish quality control procedures: The SOP should specify the procedures for performing quality control tests, such as using control strains or spikes of known microorganisms.
- Prepare quality control materials: The SOP should specify how to prepare the quality control materials, including culture media and any reagents or solutions needed.
- Perform quality control tests: The SOP should specify how to perform the quality control tests, including any specific procedures or criteria used to interpret the results.
- Record the results: The SOP should specify how to record the results of the quality control tests, including any criteria used to interpret the results.
- Troubleshoot problems: The SOP should specify how to troubleshoot any problems encountered during quality control testing, such as if results do not meet the established criteria.
- Document quality control: The SOP should specify how to document the quality control results, including any corrective actions taken if needed.
Overall, a quality control SOP is critical to ensuring that microbiology testing is performed consistently and accurately. The SOP should be followed closely to minimize the risk of errors or contamination and to ensure that the results generated are accurate and reliable.
Troubleshooting SOP:
In microbiology testing, it’s important to have a troubleshooting SOP in place to help identify and correct any issues that may arise during the testing process. Here are some general steps that may be included in a troubleshooting SOP for microbiology testing:
- Identify the problem: The SOP should specify how to identify the problem, such as through observation of abnormal results or unexpected behavior of microorganisms.
- Isolate the cause of the problem: The SOP should specify how to isolate the cause of the problem, such as through repeat testing or by reviewing the testing procedures and identifying any potential errors or issues.
- Take corrective actions: The SOP should specify the corrective actions that should be taken to address the problem, such as retesting, adjusting testing conditions, or reevaluating the test procedure.
- Document the problem and corrective actions: The SOP should specify how to document the problem and any corrective actions taken to address it, including any changes to the testing procedures.
- Follow up: The SOP should specify how to follow up to ensure that the corrective actions were successful and that the problem has been resolved.
Overall, a troubleshooting SOP is critical to minimizing errors and ensuring accurate and reliable microbiology testing results. The SOP should be followed closely to ensure that any issues that arise are identified and resolved in a timely and effective manner.
Equipment Maintenance SOP :
In microbiology testing, it is important to have an equipment maintenance SOP in place to ensure that all equipment is properly maintained and functioning correctly. Here are some general steps that may be included in an equipment maintenance SOP for microbiology testing:
- Identify equipment to be maintained: The SOP should specify which equipment requires regular maintenance, including incubators, microscopes, pipettes, and other laboratory equipment.
- Establish a maintenance schedule: The SOP should specify how often each piece of equipment should be maintained, including routine maintenance, calibration, and cleaning.
- Perform routine maintenance: The SOP should specify the routine maintenance procedures for each piece of equipment, including cleaning, lubrication, and inspection.
- Calibrate equipment: The SOP should specify how to calibrate equipment, including any tools or standards required.
- Keep records of maintenance: The SOP should specify how to keep records of maintenance for each piece of equipment, including when it was last maintained and any issues identified during maintenance.
- Troubleshoot equipment problems: The SOP should specify how to troubleshoot any problems encountered with equipment, including when and how to seek assistance if needed.
- Document equipment maintenance: The SOP should specify how to document equipment maintenance, including any corrective actions taken if issues are identified.
Overall, an equipment maintenance SOP is critical to ensuring that microbiology testing equipment is functioning properly and generating accurate and reliable results. The SOP should be followed closely to minimize the risk of equipment failure or errors, and to ensure that all equipment is well-maintained and calibrated to perform optimally.
Safety SOP:
In microbiology testing, safety is of utmost importance, and a safety SOP is necessary to ensure that laboratory personnel take appropriate precautions to minimize the risk of injury or infection. Here are some general steps that may be included in a safety SOP for microbiology testing:
- Identify potential hazards: The SOP should specify the potential hazards associated with each testing procedure, such as exposure to pathogenic microorganisms, hazardous chemicals, or physical hazards.
- Develop procedures for handling hazardous materials: The SOP should specify the procedures for handling and disposing of hazardous materials, such as pathogenic microorganisms, biological waste, or hazardous chemicals.
- Specify personal protective equipment (PPE): The SOP should specify the PPE that should be worn by laboratory personnel when performing microbiology testing, including gloves, lab coats, eye protection, and respiratory protection if necessary.
- Train laboratory personnel: The SOP should specify the training that should be provided to laboratory personnel on the potential hazards and risks associated with microbiology testing, as well as the proper procedures for handling hazardous materials and using PPE.
- Establish emergency procedures: The SOP should specify the procedures for handling emergencies, such as accidental exposure to hazardous materials or equipment failure.
- Maintain a clean and organized laboratory: The SOP should specify the procedures for maintaining a clean and organized laboratory, including procedures for cleaning equipment, work surfaces, and spills.
- Specify procedures for handling sharps: The SOP should specify the procedures for handling and disposing of sharps, such as needles or lancets, to minimize the risk of injury and infection.
- Monitor laboratory conditions: The SOP should specify procedures for monitoring laboratory conditions, such as temperature and humidity, to ensure that they are within acceptable limits.
Overall, a safety SOP is critical to ensuring the health and safety of laboratory personnel, as well as the accuracy and reliability of microbiology testing results. The SOP should be followed closely to minimize the risk of injury or infection and to ensure that all laboratory personnel are aware of the potential hazards associated with microbiology testing.
Data recording and analysis SOP:
Data recording and analysis SOP is an essential component of microbiology testing, as it ensures that all data generated during testing is accurate, complete, and reliable. Here are some general steps that may be included in a data recording and analysis SOP for microbiology testing:
- Specify the data to be recorded: The SOP should specify the data that needs to be recorded for each testing procedure, such as sample identification, testing parameters, and results.
- Identify the data recording method: The SOP should specify the method of data recording, such as electronic or paper-based, and provide guidelines for ensuring the accuracy and completeness of data.
- Establish procedures for data management: The SOP should specify the procedures for managing data, including procedures for data entry, storage, and retrieval.
- Develop procedures for data analysis: The SOP should specify the procedures for analyzing data, including the use of appropriate statistical techniques and software.
- Identify personnel responsible for data management and analysis: The SOP should identify the personnel responsible for data management and analysis and specify their roles and responsibilities.
- Develop procedures for data review and approval: The SOP should specify the procedures for reviewing and approving data, including the use of appropriate controls and checks.
- Establish procedures for data retention: The SOP should specify the procedures for retaining data, including the duration of data retention and the method of data disposal.
Overall, a data recording and analysis SOP is essential for ensuring the accuracy, completeness, and reliability of microbiology testing results. The SOP should be followed closely to ensure that all data is recorded and analyzed accurately and that appropriate controls are in place to ensure the integrity of the data.
Reporting of results SOP:
Reporting of results SOP is an essential component of microbiology testing, as it ensures that all results generated during testing are accurately and effectively communicated to relevant stakeholders. Here are some general steps that may be included in a reporting of results SOP for microbiology testing:
- Specify the format and content of reports: The SOP should specify the format and content of reports, including the required information such as test name, test results, interpretation, and any necessary comments or recommendations.
- Identify the personnel responsible for generating reports: The SOP should identify the personnel responsible for generating reports, specify their roles and responsibilities, and ensure that they are adequately trained.
- Establish procedures for reviewing and approving reports: The SOP should specify the procedures for reviewing and approving reports, including any necessary checks and controls.
- Develop procedures for the distribution of reports: The SOP should specify the procedures for distributing reports to relevant stakeholders, such as the requesting physician, patient, or regulatory agencies.
- Identify the timelines for reporting: The SOP should specify the timelines for reporting results to ensure that reports are provided within the required timeframe.
- Establish procedures for reporting of critical or urgent results: The SOP should specify the procedures for reporting critical or urgent results, including the persons responsible for communicating the results and the timelines for communication.
Overall, a reporting of results SOP is essential for ensuring that the results of microbiology testing are effectively communicated to relevant stakeholders. The SOP should be followed closely to ensure that reports are generated accurately, reviewed and approved promptly, and distributed to stakeholders within the required timeframe.
Review and approval SOP:
Review and approval SOP is an important component of microbiology testing, as it ensures that all testing procedures, results, and reports are reviewed and approved by authorized personnel. Here are some general steps that may be included in a review and approval SOP for microbiology testing:
- Specify the personnel authorized to review and approve: The SOP should specify the personnel authorized to review and approve testing procedures, results, and reports, such as supervisors, managers, or quality control personnel.
- Establish procedures for review and approval: The SOP should specify the procedures for review and approval, including the required level of review, timelines for review and approval, and any necessary documentation.
- Identify the criteria for review and approval: The SOP should identify the criteria for review and approval, such as compliance with regulatory requirements, adherence to standard operating procedures, and accuracy of results.
- Develop procedures for documenting review and approval: The SOP should specify the procedures for documenting the review and approval process, including the identification of the reviewer, the date of review and approval, and any comments or feedback provided.
- Establish procedures for resolving issues identified during review: The SOP should establish procedures for resolving any issues identified during the review process, including any necessary corrective and preventive actions.
Overall, a review and approval SOP is essential for ensuring the quality and accuracy of microbiology testing procedures, results, and reports. The SOP should be followed closely to ensure that all testing procedures are reviewed and approved by authorized personnel, and that any issues identified during the review process are addressed promptly and appropriately.
Disposal of Samples and Materials SOP:
Disposal of samples and materials SOP is an essential component of microbiology testing, as it ensures that all samples and materials are disposed of safely and in compliance with regulatory requirements. Here are some general steps that may be included in a disposal of samples and materials SOP for microbiology testing:
- Identify the types of samples and materials that require disposal: The SOP should identify the types of samples and materials that require disposal, including contaminated media, cultures, and equipment.
- Establish procedures for segregation of waste: The SOP should establish procedures for segregating waste, such as the use of color-coded bags or containers, to ensure that different types of waste are properly separated.
- Develop procedures for packaging and labeling waste: The SOP should specify the procedures for packaging and labeling waste, including the use of biohazard labels and the proper disposal of sharps.
- Identify the appropriate disposal methods: The SOP should identify the appropriate disposal methods for different types of waste, such as incineration, autoclaving, or chemical disinfection.
- Develop procedures for documentation: The SOP should specify the procedures for documenting waste disposal, including the identification of the person responsible for disposal, the date and time of disposal, and any necessary documentation.
- Establish procedures for monitoring and verification: The SOP should establish procedures for monitoring and verifying that waste is disposed of properly, such as regular inspections and audits.
Overall, a disposal of samples and materials SOP is essential for ensuring the safe and compliant disposal of waste generated during microbiology testing. The SOP should be followed closely to ensure that all waste is properly segregated, packaged, labeled, and disposed of according to regulatory requirements and best practices.
Training and implementation SOP:
A training and implementation SOP is crucial for ensuring that all personnel involved in microbiology testing are properly trained and competent to perform their roles. Here are some general steps that may be included in a training and implementation SOP for microbiology testing:
- Identify the personnel who require training: The SOP should identify the personnel who require training, including laboratory staff, supervisors, and any other personnel involved in microbiology testing.
- Develop training programs: The SOP should specify the training programs required for each role, including general laboratory safety, aseptic techniques, media preparation, testing procedures, quality control, and data analysis.
- Establish procedures for training delivery: The SOP should establish procedures for the delivery of training, including the use of online courses, classroom sessions, or one-on-one training.
- Develop procedures for competency assessment: The SOP should specify procedures for assessing the competency of personnel following training, including practical assessments and written exams.
- Establish procedures for ongoing training and retraining: The SOP should establish procedures for ongoing training and retraining, including the identification of training needs, the development of training programs, and the delivery of training.
- Develop procedures for documentation: The SOP should specify procedures for documenting training and competency assessments, including the identification of personnel who have completed training and the results of competency assessments.
- Establish procedures for implementation: The SOP should establish procedures for implementing the training and competency assessment programs, including the identification of responsible personnel, timelines for implementation, and ongoing monitoring and evaluation.
Overall, a training and implementation SOP is essential for ensuring that all personnel involved in microbiology testing are properly trained and competent to perform their roles. The SOP should be followed closely to ensure that all personnel receive the necessary training and that their competency is regularly assessed and monitored.
Record keeping SOP:
A record-keeping SOP is essential in microbiology testing to ensure that all data and information generated during testing are accurately and completely recorded, stored, and easily retrievable. Here are some general steps that may be included in a record-keeping SOP for microbiology testing:
- Identify the records to be maintained: The SOP should identify the types of records that need to be maintained, such as sample information, test results, quality control records, equipment maintenance records, and personnel training records.
- Establish procedures for record creation and retention: The SOP should specify procedures for creating and retaining records, including the formats and templates to be used, the frequency of record creation, and the duration of record retention.
- Develop procedures for data entry: The SOP should specify procedures for data entry, including the use of electronic or manual data entry, the use of standard abbreviations and terminology, and the verification of data accuracy.
- Establish procedures for record storage: The SOP should specify procedures for record storage, including the use of secure storage areas, access controls, and backup procedures.
- Develop procedures for record retrieval: The SOP should specify procedures for record retrieval, including the identification of responsible personnel, access controls, and record retrieval timelines.
- Establish procedures for record review and approval: The SOP should specify procedures for record review and approval, including the identification of responsible personnel, timelines for review, and procedures for addressing any discrepancies or errors.
- Develop procedures for record disposal: The SOP should specify procedures for record disposal, including the use of secure disposal methods and timelines for disposal.
Overall, a record-keeping SOP is essential in microbiology testing to ensure that all data and information are accurately recorded, stored, and easily retrievable. The SOP should be followed closely to ensure that all records are created, retained, and disposed of in accordance with established procedures.
References:
- Clinical and Laboratory Standards Institute (CLSI). (2018). Development of in vitro susceptibility testing criteria and quality control parameters. CLSI document M23, 4th ed. Wayne, PA: Clinical and Laboratory Standards Institute.
- World Health Organization. (2003). Laboratory biosafety manual (3rd ed.). Geneva, Switzerland: World Health Organization.
- U.S. Food and Drug Administration. (2016). Bacteriological analytical manual (BAM). Silver Spring, MD: U.S. Food and Drug Administration.
- American Society for Microbiology. (2019). Guidelines for biosafety in teaching laboratories (2nd ed.). Washington, DC: American Society for Microbiology.
- European Society of Clinical Microbiology and Infectious Diseases. (2018). European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Växjö, Sweden: European Society of Clinical Microbiology and Infectious Diseases.
- Centers for Disease Control and Prevention. (2015). Laboratory biosafety guidelines (3rd ed.). Atlanta, GA: Centers for Disease Control and Prevention.
- National Institutes of Health. (2019). Guidelines for research involving recombinant or synthetic nucleic acid molecules (NIH Guidelines). Bethesda, MD: National Institutes of Health.
- International Organization for Standardization. (2017). ISO 15189:2017 Medical laboratories – Requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization.
- United States Environmental Protection Agency. (2018). Biosafety in microbiological and biomedical laboratories (BMBL) (5th ed.). Washington, DC: United States Environmental Protection Agency.
- American Industrial Hygiene Association. (2019). Laboratory safety guidelines (3rd ed.). Falls Church, VA: American Industrial Hygiene Association.
Possible References Used