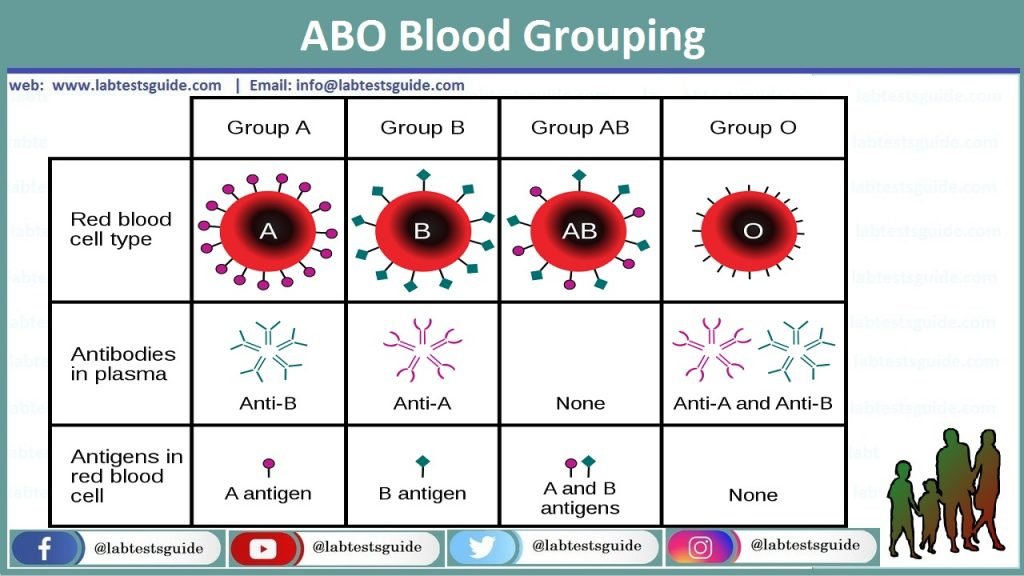
ABO grouping is a test performed to determine an individual’s blood type. It is based on the premise that individuals have antigens on their red blood cells (RBCs) that correspond to the four main blood groups: A, B, O, and AB.
The purpose is to detect the presence or absence of A and B antigens on red cells by using Anti-A and anti-B antibodies against the corresponding antigens. These Anti-A and anti-B are monoclonal origin. The antibodies, of immunoglobulin class IgM, are specific against red cells antigen. Reverse typing shall always be run in parallel with ABO typing. Antibodies present in patient’s serum react with reagent red cells. This reaction indicates presence or absence of antibody.
PRINCIPLE:
Front/Forward type: Direct agglutination of red cells with a particular reagent indicates the presence of the corresponding antigen. No agglutination indicates its absence. The ABO group of red cells is determined from the pattern of reactivity obtained with test reagents.
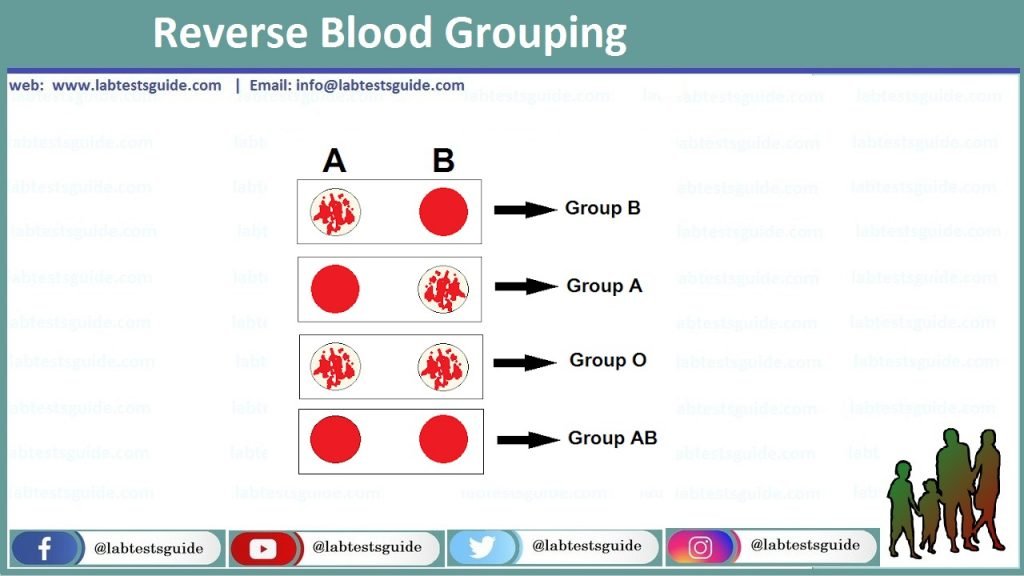
Back/Reverse type: Antibodies present in patient’s serum reacts with the antigen on red cell. Agglutination is suggestive of presence of corresponding antigen and no agglutination indicates absence of antibody.
NOTE: Patient’s who need only ABO/Rh performed shall have ‘O’ cells in their back type as screen for Bombay phenotype.
EQUIPMENT, MATERIALS AND REAGENTS:
- 12×75 mm test tubes
- Test tube rack
- Transfer pipettes
- Marking pens
- Isotonic saline
- Serologic centrifuge
- Lighted Agglutination viewer
- DiaClon Anti-A
- DiaClon Anti-B
- A1 cells
- B cells
- O cells
PRECAUTIONS:
- For in vitro diagnosis only.
- Store at 2-8oC Do not freeze.
- Turbidity may indicate reagent deterioration or contamination.
- Do not use beyond expiry date?
- All reagents shall be considered as potentially hazardous.
SPECIMEN COLLECTION:
Red cells from the EDTA anti-coagulated are best suited for front type and clotted sample is suitable for back typing. Samples can be tested up to 3 days from the date of collection.
QUALITY CONTROL:
To confirm the reactivity of anti-sera, it is recommended that these reagents be tested each day of use with antigen positive and negative red cells. Refer to quality control procedures for detail.
PROCEDURE:
- Prepare 5% suspension of patient’s red cells.
- Centrifuge the clotted specimen that clears serum is obtained.
- Label 4 test tube with patient’s complete MR#/OPD# or donor#.
FRONT TYPE:
- Tube ‘A’ for Anti-A
- Tube ‘B’ for Anti-B
BACK TYPE:
- Tube ‘ a1 ’for A1 cells
- Tube ‘ b ’ for B cells
- Add 1 drop (50ul) of each blood-grouping reagent to tube ‘A’ and ‘B’ and add 2 drops of patient’s serum in tubes ‘a1’ and ‘b’.
- Now add 1 drop of patient’s red cell 5% suspension to tube ‘A’ and ‘B’.
- Place 1 drop of reagent A1 and B reagent cells into tubes ‘a1’ and ‘b’.
- Mix the contents of each tube thoroughly and centrifuge immediately at calibrated speed and time.
- Gently re-suspend red cell buttons. Examine for agglutination macroscopically under agglutination viewer.
- Grade and record results in the medical software. For additional software options visit https://www.foreseemed.com/hcc-risk-adjustment-coding.
NOTE: Patient’s who need only ABO/Rh performed shall have ‘O’ cells in their back type as screen for Cold type antibodies, auto-antibodies and Bombay phenotype.
- If patient group is ‘O’ then label another tube for ‘O’ cells. Add 2 drops of patient’s serum in the tube and 1 drop of ‘O’ cells in it and then proceed from the step #7 to step#8.
Results are interpreted as
RESULTS:
POSITIVE RESULT: Hemolysis /agglutination of red cells
NEGATIVE RESULT: No hemolysis/no agglutination of red cells.
INTERPRETATION:
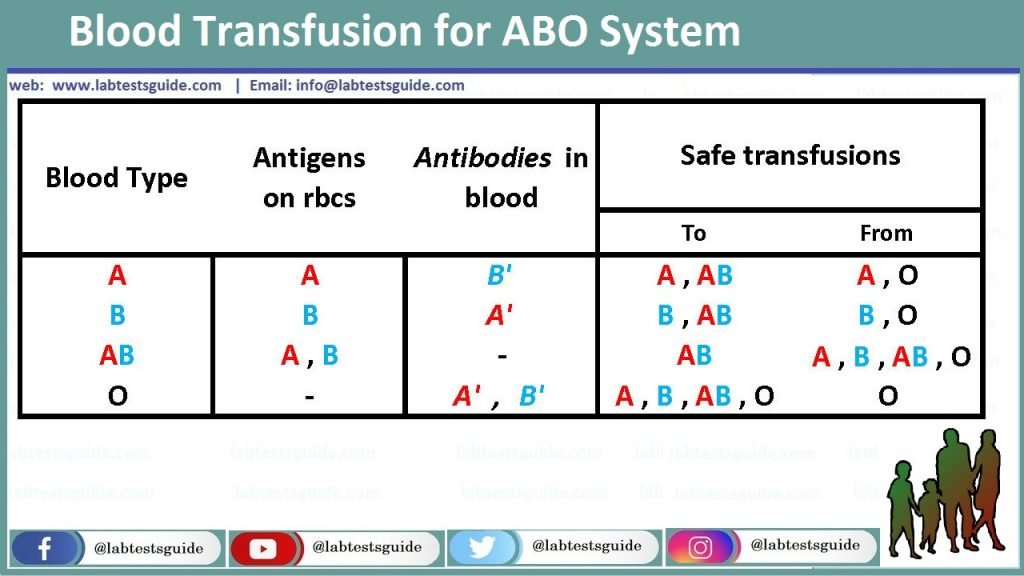
| Anti-A | Anti-B | ‘A1’ Cells | ‘B’ cells | ‘O’ cells | Blood group |
| ++++ | +++ | A | |||
| ++++ | +++ | B | |||
| +++ | +++ | O | |||
| ++++ | ++++ | AB | |||
| +++ | +++ | +++ | Possible Bombay type* |
- Whenever a blood group is ‘AB Positive’ run an Rh control. See Rh blood grouping procedure for method.
- Suspected Bombay phenotype will be confirmed after testing of Anti-H with patient’s cells.
ABO DISCREPANCIES:
- A discrepancy exists when the results of the front type do not match the back type.
- When a discrepancy is encountered, results should be recorded but Interpretation must be delayed until the discrepancy is resolved.
Refer to ABO discrepancy procedure for detail about etiology and resolution.
TECHNICAL PROBLEMS:
Any technical problem giving falsely positive and falsely negative results may be encountered due to following reasons:
| No. | False Negative | False positive |
| Centrifugation time too short | Over-centrifugation of tubes | |
| 2. | Incorrect interpretation or recording of results | Incorrect interpretation or recording of results |
| 3. | Inappropriate ratio of serum/reagent to cells | Use of dirty glassware |
| 4. | Hemolysis not identified as positive reaction | Use of contaminated reagents, cells or saline |
| 5. | Test incubated at temperature above than 20-24oC | Cells contaminated with ‘Wharton’s jelly’ |
| 6. | Reagent or serum not added to tube |
STABILITY OF REACTIONS:
Following centrifugation all the tubes should be read immediately and results interpreted without delay. Delay may result in dissociation of antigen-antibody complex leading to falsely negative or weak positive results.
Related Articles:
Possible References Used




