Gel electrophoresis is a method used in the laboratory to separate DNA, RNA, or proteins from each other. Molecules of interest are forced through a porous gel using an electric current, with one end of the gel positively charged and the other end negatively charged. This causes negatively charged molecules, such as DNA and RNA, to move toward the positive end of the gel. Since proteins can have various charges, they must be neutralized using sodium dodecyl sulfate (SDS) to ensure that the separation of molecules is not affected by charge but only by size. SDS also denatures proteins, preventing variations in the shape of molecules from affecting migration patterns.
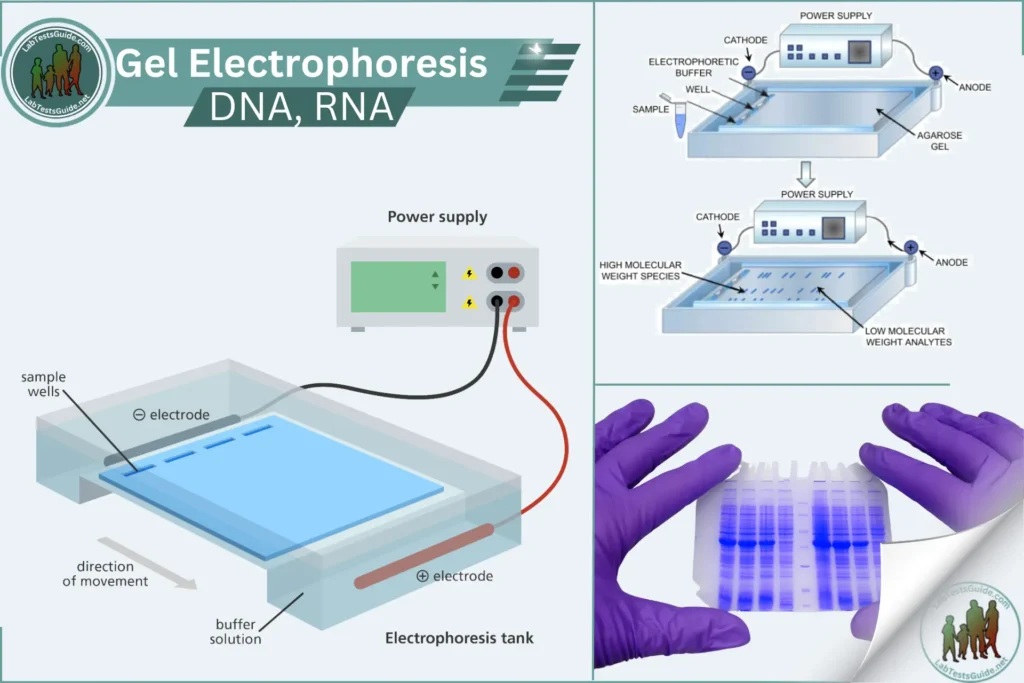
Due to the pore size of the gel, larger molecules do not travel as far as smaller molecules, allowing separation in size. Ultimately, the separated molecules can be visualized as bands.
Introduction
Gel electrophoresis is a technique used to separate DNA, RNA, or proteins based on their size and charge. This method is widely used in molecular biology for analyzing the composition of nucleic acids and proteins.
Principle
Molecules are separated by applying an electric field to a gel matrix. Smaller molecules move faster through the gel, while larger molecules move slower, allowing for size-based separation. DNA and RNA are separated on agarose gels, while proteins are separated on polyacrylamide gels (PAGE).
Specimen Requirements
- Type of specimen: DNA, RNA, or protein samples
- Volume of specimen required: Typically 5-10 µL per well
- Collection method: Standard extraction protocols for nucleic acids or proteins
- Handling and storage requirements: Store samples at -20°C or -80°C for long-term storage
- Stability of the specimen: Stable for several months at -20°C or -80°C
Reagents and Materials
- Agarose or polyacrylamide powder
- Electrophoresis buffer (e.g., TAE, TBE for nucleic acids; SDS-PAGE buffer for proteins)
- DNA/RNA loading dye or protein loading buffer
- Ethidium bromide or SYBR Safe for DNA/RNA staining
- Coomassie Brilliant Blue or silver stain for protein staining
- DNA/RNA ladder or protein marker
- Gel casting tray and comb
- Power supply
- Electrophoresis chamber
- Distilled water
Equipment
- Gel electrophoresis apparatus (gel box and power supply)
- UV transilluminator or gel documentation system
- Pipettes and tips
- Microwave or hot plate (for melting agarose)
- Gel documentation system (for imaging)
Procedure
Agarose Gel Electrophoresis (for DNA/RNA):
- Prepare the Gel:
- Weigh out the appropriate amount of agarose powder (e.g., 1% agarose for general DNA separation).
- Dissolve the agarose in electrophoresis buffer (e.g., TAE or TBE) by heating in a microwave or on a hot plate until fully melted.
- Allow the solution to cool slightly before pouring it into the gel casting tray with a comb to create wells.
- Let the gel solidify at room temperature.
- Prepare the Samples:
- Mix the DNA/RNA samples with loading dye.
- Load the samples and a DNA/RNA ladder into the wells of the gel.
- Run the Gel:
- Place the gel in the electrophoresis chamber and cover it with electrophoresis buffer.
- Connect the chamber to the power supply and run the gel at a constant voltage (e.g., 100-150V) until the dye has migrated an appropriate distance.
- Stain the Gel:
- Stain the gel with ethidium bromide or SYBR Safe (if not already included in the gel) to visualize the DNA/RNA.
- View the gel under a UV transilluminator or gel documentation system.
Polyacrylamide Gel Electrophoresis (SDS-PAGE) (for Proteins):
- Prepare the Gel:
- Assemble the gel casting apparatus.
- Prepare the resolving gel solution and pour it into the gel casting apparatus. Allow it to polymerize.
- Prepare the stacking gel solution and pour it on top of the resolving gel. Insert a comb to create wells and allow it to polymerize.
- Prepare the Samples:
- Mix protein samples with SDS-PAGE loading buffer and heat to denature the proteins.
- Load the samples and a protein marker into the wells of the gel.
- Run the Gel:
- Place the gel in the electrophoresis chamber and cover it with SDS-PAGE running buffer.
- Connect the chamber to the power supply and run the gel at a constant voltage (e.g., 100-200V) until the dye front reaches the bottom of the gel.
- Stain the Gel:
- Remove the gel from the apparatus and stain with Coomassie Brilliant Blue or silver stain to visualize the proteins.
- Destain the gel to remove excess stain and enhance visibility of the protein bands.
Quality Control
- Include a DNA/RNA ladder or protein marker to estimate the size of separated molecules.
- Run positive and negative control samples to ensure the accuracy and reliability of the results.
Calculation and Interpretation
- Compare the migration distance of sample bands to the ladder/marker to determine the size of the molecules.
- Analyze the band patterns to interpret the results, considering factors such as band intensity and position.
Limitations
- Overloading wells can cause poor resolution.
- Gel concentrations must be optimized for the size range of interest.
- Staining procedures must be carefully controlled to avoid inconsistent results.
Safety Precautions
- Wear appropriate PPE (gloves, lab coat, safety goggles).
- Handle ethidium bromide and other stains with care as they are hazardous.
- Use UV protection when viewing gels under UV light.
- Dispose of waste according to local regulations.
References
- Sambrook, J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual (3rd ed.).
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680-685.
- Manufacturer’s instructions for electrophoresis reagents and equipment.
- Clinical Laboratory Standards Institute (CLSI) guidelines.
This format can be adapted for various types of gel electrophoresis by adjusting the specific details to match the methodology used for each type.
Possible References Used