Cystine Tryptic Agar (CTA) is a semisolid growth medium used for the isolation and cultivation of fastidious microorganisms. It is a versatile medium that can also be used for detecting bacterial motility, performing fermentation tests, and conducting antimicrobial susceptibility testing. CTA is an important tool for microbiologists and other healthcare professionals who work to diagnose and treat infectious diseases.
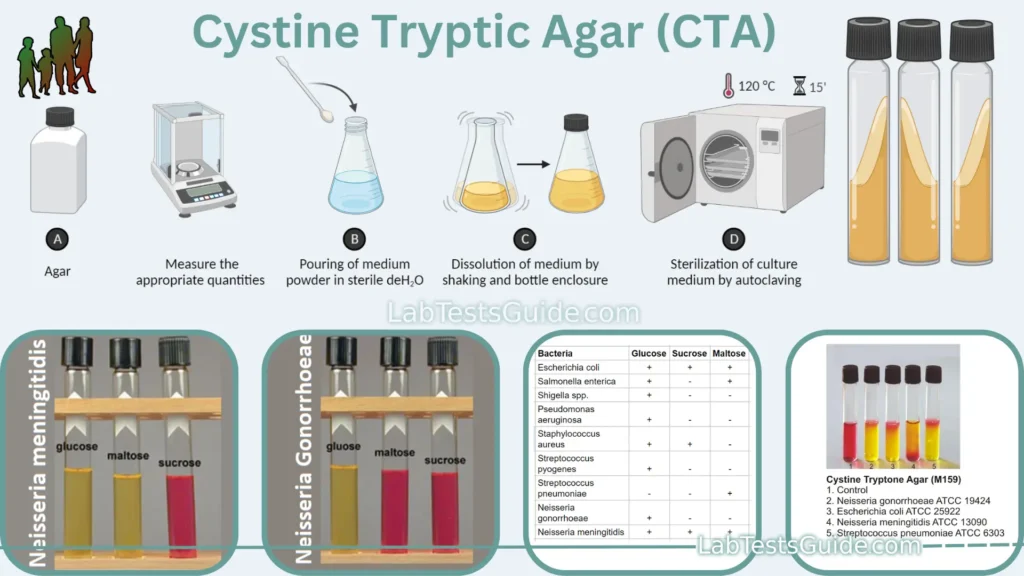
CTA is a semi-solid medium that contains cystine, casein peptone, sodium chloride, sodium sulfite, and agar. Cystine and casein peptone provide essential nutrients for the growth of fastidious microorganisms. Sodium chloride maintains the osmotic balance of the medium, and sodium sulfite inhibits the growth of other bacteria that produce hydrogen sulfide. Agar gives the medium a semisolid consistency, which is ideal for testing bacterial motility and performing fermentation tests.
Key Points of Cystine Tryptic Agar:
- CTA is a semi-solid growth medium used for the isolation, cultivation, and identification of fastidious microorganisms, such as Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae.
- CTA contains cystine and casein peptone, which provide essential nutrients for the growth of fastidious microorganisms.
- CTA also contains sodium chloride, sodium sulfite, and agar. Sodium chloride maintains the osmotic balance of the medium, sodium sulfite inhibits the growth of other bacteria that produce hydrogen sulfide, and agar gives the medium a semisolid consistency.
- CTA is a versatile medium that can be used for a variety of purposes, including:
- Isolation and cultivation of fastidious microorganisms
- Detection of bacterial motility
- Performance of fermentation tests
- Antimicrobial susceptibility testing
- To test bacterial motility, a stab inoculation is performed. If the bacterium is motile, it will spread out from the stab line.
- To perform a fermentation test, the CTA medium is supplemented with a specific carbohydrate. If the bacterium can ferment the carbohydrate, it will produce acid, which will cause the phenol red pH indicator in the medium to change color from red to yellow.
- CTA is a valuable tool for microbiologists and other healthcare professionals who need to isolate, cultivate, and identify fastidious microorganisms.
- CTA is typically incubated at 35-37°C in a humidified atmosphere for 18-24 hours.
- CTA plates should be examined for growth and any changes in the color of the medium.
- If growth is observed, the organism can be identified using a variety of methods, such as Gram staining, biochemical tests, and serological tests.
- CTA plates can also be used to perform antimicrobial susceptibility testing.
- Isolation of Neisseria gonorrhoeae: CTA is used to isolate Neisseria gonorrhoeae from clinical specimens, such as urethral swabs and cervical swabs.
- Detection of Neisseria meningitidis: CTA is used to detect Neisseria meningitidis in the cerebrospinal fluid of patients with meningitis.
- Identification of Streptococcus pneumoniae: CTA can be used to identify Streptococcus pneumoniae by its ability to ferment inulin.
- Performance of fermentation tests: CTA can be used to perform fermentation tests on a variety of bacteria, including Enterobacteriaceae, non-fermenting Gram-negative bacteria, and Gram-positive cocci.
- Antimicrobial susceptibility testing: CTA plates can be used to perform antimicrobial susceptibility testing on a variety of bacteria.
Defination of Cystine Tryptic Agar:
Cystine Tryptic Agar (CTA) is a semi-solid growth medium used for the isolation, cultivation, and identification of fastidious microorganisms, such as Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. It can also be used to detect bacterial motility and to perform fermentation tests.
History and Modifications of Cystine Tryptic Agar:
Development of CTA (1916):
- CTA was first developed in 1916 by American bacteriologist John H. Mueller.
- Developed while Mueller was at the Rockefeller Institute for Medical Research.
- Designed to cultivate the fastidious bacterium Neisseria gonorrhoeae.
Original CTA Formulation:
- Mueller’s original CTA formulation comprised cystine, tryptose, sodium chloride, and agar.
- Supported the growth of Neisseria gonorrhoeae and other fastidious microorganisms.
Modifications of CTA: CTA has undergone various modifications over time to enhance its utility:
- Addition of Sodium Sulfite:
- Significant modification involved adding sodium sulfite.
- Sodium sulfite inhibits the growth of hydrogen sulfide-producing bacteria, such as Proteus spp.
- Increased selectivity, particularly for isolating Neisseria gonorrhoeae and other fastidious microorganisms.
- Addition of Carbohydrates:
- Carbohydrates can be added to CTA for fermentation tests.
- Fermentation of carbohydrates leads to acid production, causing a color change in the phenol red pH indicator (red to yellow).
- Addition of Antibiotics:
- Antibiotics may be incorporated into CTA for antimicrobial susceptibility testing.
- Susceptible bacteria do not grow in the presence of the antibiotic.
- Addition of Blood:
- Blood supplementation improves the growth of certain fastidious microorganisms, e.g., Haemophilus influenzae.
These modifications make CTA a versatile medium that can be adapted to various microbiological applications, from selective isolation to biochemical testing and antimicrobial susceptibility assessments.
Purpose and Significance of Cystine Tryptic Agar:
Purpose:
- Isolation and Cultivation of Fastidious Microorganisms: CTA is designed for the isolation, cultivation, and growth of fastidious microorganisms, which require specific nutrients or environmental conditions to thrive.
- Versatility for Microbial Studies: CTA serves as a versatile medium with multiple applications, including the isolation and cultivation of various fastidious microorganisms.
- Detection of Bacterial Motility: It can be utilized to assess bacterial motility through stab inoculations, helping to identify motile microorganisms by their spread from the stab line.
- Fermentation Testing: CTA can be supplemented with specific carbohydrates to conduct fermentation tests. Acid production during carbohydrate fermentation leads to a change in the pH indicator’s color, aiding in bacterial identification.
- Antimicrobial Susceptibility Testing: CTA plates are suitable for conducting antimicrobial susceptibility testing, which is vital for determining the susceptibility or resistance of bacteria to antibiotics.
Significance:
- Reliable and Consistent Results: CTA is known for its reliability, producing consistent results. This consistency is crucial for accurate bacterial identifications and diagnoses in clinical and research settings.
- Cost-Effective Medium: CTA’s relatively low cost makes it accessible to a wide range of laboratories, including those in resource-limited or developing countries, contributing to the global availability of microbiological tools.
- Isolation of Pathogens: In clinical microbiology, CTA plays a significant role in isolating and identifying pathogens such as Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae from clinical specimens.
- Meningitis Detection: It aids in detecting Neisseria meningitidis in cerebrospinal fluid samples from patients with meningitis, facilitating timely diagnoses and treatment.
- Identification of Specific Bacteria: CTA can be used to identify bacteria based on their ability to ferment specific carbohydrates, such as inulin for Streptococcus pneumoniae.
- Fermentation Testing: CTA is instrumental in conducting fermentation tests for various bacterial groups, including Enterobacteriaceae, non-fermenting Gram-negative bacteria, and Gram-positive cocci.
- Antimicrobial Susceptibility Testing: Its use in antimicrobial susceptibility testing helps guide clinicians in selecting the most effective antibiotics for treating infections, thereby improving patient care.
Importance of Cystine Tryptic Agar in Microbiology:
Versatility in Microbiological Applications:
- Isolation and Cultivation of Fastidious Microorganisms: CTA is instrumental in isolating and cultivating fastidious microorganisms that have specific nutritional requirements.
- Detection of Bacterial Motility: It allows for the detection of bacterial motility, aiding in the identification of motile microorganisms through stab inoculation.
- Fermentation Testing: CTA can be customized with specific carbohydrates for conducting fermentation tests, which help identify microorganisms based on their ability to ferment carbohydrates and produce acid.
- Antimicrobial Susceptibility Testing: CTA plates are suitable for conducting antimicrobial susceptibility testing, a critical step in determining the effectiveness of antibiotics against specific bacterial strains.
Reliability and Consistency:
- Consistent Results: CTA is known for its reliability, consistently producing accurate results. This consistency is vital for making precise bacterial identifications and diagnoses, crucial in clinical and research contexts.
Cost-Effectiveness and Accessibility:
- Affordable Medium: CTA’s relatively low cost makes it accessible to a wide range of laboratories, including those in resource-limited or developing countries, contributing to the global availability of microbiological tools.
Clinical Microbiology Applications:
- Isolation of Pathogens: CTA plays a significant role in isolating and identifying pathogens like Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae from clinical specimens.
- Meningitis Detection: It is used to detect Neisseria meningitidis in cerebrospinal fluid samples from patients with meningitis, aiding in timely diagnoses and treatment.
- Identification of Specific Bacteria: CTA facilitates the identification of bacteria based on their fermentation capabilities, as seen with Streptococcus pneumoniae’s ability to ferment inulin.
- Fermentation Testing: CTA supports fermentation tests for various bacterial groups, including Enterobacteriaceae, non-fermenting Gram-negative bacteria, and Gram-positive cocci.
- Antimicrobial Susceptibility Testing: It is a valuable tool in determining bacterial susceptibility or resistance to antibiotics, assisting clinicians in selecting effective treatment strategies.
Short Overview about Neisseria Spp:
Neisseria spp. are a group of bacteria belonging to the Neisseriaceae family, which includes both pathogenic and non-pathogenic species. They are Gram-negative cocci, typically occurring in pairs (diplococci) under the microscope. Here’s a brief overview of Neisseria spp.:
- Pathogenic Species: The two most clinically significant Neisseria species are Neisseria gonorrhoeae and Neisseria meningitidis.
- Neisseria gonorrhoeae: This bacterium is the causative agent of gonorrhea, a sexually transmitted infection. It primarily infects the genital and rectal mucous membranes and can lead to various complications if left untreated.
- Neisseria meningitidis: Commonly known as the meningococcus, this bacterium can cause invasive infections such as meningitis and septicemia. It can lead to serious and life-threatening diseases, particularly in crowded settings.
- Non-Pathogenic Species: Many other Neisseria species are part of the normal human microbiota and are considered non-pathogenic. They can be found in various mucous membranes, including the oral and respiratory tracts.
- Colonization: Some Neisseria species, like Neisseria lactamica, are early colonizers of the human nasopharynx and may play a role in developing natural immunity to more pathogenic species.
- Gram-Negative: Neisseria spp. are Gram-negative bacteria, meaning they have a cell wall composed of a thin peptidoglycan layer and an outer membrane.
- Diplococci: They typically appear as pairs of cocci (round cells) when viewed under a microscope.
- Transmission: Pathogenic Neisseria species are primarily transmitted through close contact, such as sexual contact for N. gonorrhoeae or respiratory droplets for N. meningitidis.
- Vaccination: Vaccines are available for some strains of Neisseria meningitidis to prevent meningococcal infections. No vaccine is currently available for Neisseria gonorrhoeae.
- Antibiotic Resistance: Both N. gonorrhoeae and some strains of N. meningitidis have developed antibiotic resistance, making treatment challenging in some cases.
- Diagnosis: Diagnosis of Neisseria infections typically involves culture and laboratory testing of clinical specimens, such as swabs or cerebrospinal fluid.
- Public Health Concern: Neisseria meningitidis outbreaks can be a significant public health concern, especially in crowded settings like college dormitories or military barracks.
In summary, Neisseria spp. encompass a group of bacteria, with N. gonorrhoeae and N. meningitidis being the most medically relevant. While some species are part of the normal human microbiota, pathogenic Neisseria can cause serious infections and pose public health challenges.
Principles of Cystine Tryptic Agar:
1. Nutrient Supply: CTA contains cystine and casein peptone, providing essential nutrients to support the growth of fastidious microorganisms, which have specific nutritional requirements.
2. Fermentation Detection: When CTA is supplemented with a specific carbohydrate, it serves as a medium to detect fermentation reactions. Bacterial metabolism of carbohydrates produces organic acids, leading to a decrease in pH and a color change in the medium.
3. pH Indicator: Phenol red is used as a pH indicator in CTA. As bacteria ferment carbohydrates and produce acids, the medium becomes acidified, causing a color shift from red-pink to yellow.
4. Peptone Degradation: Peptone in the medium is also degraded by bacteria, yielding alkaline end products. The phenol red indicator changes to yellow when the acid produced by carbohydrate fermentation surpasses the alkaline end products of peptone degradation, occurring around pH 6.8.
5. Agar for Motility Testing: The addition of agar to CTA provides a semisolid consistency, enabling the detection of bacterial motility. Motile bacteria extend from the stab line, creating turbidity throughout the medium, while non-motile organisms grow only along the stab line, leaving the surrounding medium clear.
6. Essential Nutrients: Cystine, a sulfur-containing amino acid, and casein peptone supply amino acids, vitamins, and minerals necessary for bacterial growth, particularly for fastidious microorganisms like Neisseria spp., Streptococcus pneumoniae, and Haemophilus influenzae.
7. Osmotic Balance: Sodium chloride in CTA helps maintain the osmotic balance, ensuring proper water exchange between bacterial cells and the environment, preventing cell shrinkage or swelling.
8. Inhibition of Hydrogen Sulfide Production: Sodium sulfite inhibits the growth of bacteria that produce toxic hydrogen sulfide by converting it to less toxic thiosulfate.
Clinical Applications of Cystine Tryptic Agar:
Cystine Tryptic Agar (CTA) has several clinical applications in microbiology, making it a valuable tool for diagnosing and treating infectious diseases. Here’s a summary of its clinical applications:
1. Isolation and Cultivation of Fastidious Microorganisms:
- CTA is a versatile medium for the isolation and cultivation of fastidious microorganisms. It supports the growth of bacteria that have specific nutritional requirements.
- Clinical examples include Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae.
2. Detection of Bacterial Motility:
- CTA can be used to detect bacterial motility by performing a stab inoculation.
- Motile bacteria spread out from the stab line, creating turbidity throughout the medium.
- This helps identify motile microorganisms.
3. Fermentation Tests:
- CTA can be supplemented with specific carbohydrates to perform fermentation tests.
- Bacterial fermentation of carbohydrates leads to acid production, causing a change in the medium’s color from red to yellow (phenol red pH indicator).
- These tests aid in identifying different types of bacteria based on their carbohydrate utilization patterns.
4. Antimicrobial Susceptibility Testing:
- CTA plates can be used for antimicrobial susceptibility testing.
- Antibiotic-impregnated discs are placed on the CTA plate, and the plate is incubated.
- If the bacterium is susceptible to the antibiotic, it will not grow in the area around the disc.
- This information guides the treatment of infections, helping select the most effective antibiotics.
Specific Clinical Applications:
- Isolation of Neisseria gonorrhoeae: CTA is used to isolate Neisseria gonorrhoeae from clinical specimens, such as urethral swabs and cervical swabs, aiding in the diagnosis of gonorrhea, a sexually transmitted infection.
- Detection of Neisseria meningitidis: CTA is employed to detect Neisseria meningitidis in cerebrospinal fluid samples from patients with meningitis. Neisseria meningitidis is the causative agent of meningitis, a serious infection of the meninges (membranes surrounding the brain and spinal cord).
- Identification of Streptococcus pneumoniae: CTA can identify Streptococcus pneumoniae by its ability to ferment inulin, a carbohydrate not fermented by many other bacteria. Streptococcus pneumoniae causes pneumonia, a lung infection.
- Performance of Fermentation Tests: CTA can be used for fermentation tests on various bacteria, including Enterobacteriaceae, non-fermenting Gram-negative bacteria, and Gram-positive cocci, aiding in bacterial identification.
- Antimicrobial Susceptibility Testing: CTA plates help determine bacterial susceptibility or resistance to antibiotics, guiding appropriate treatment strategies.
Ingredients, Materials and Composition of CTA:
Cystine Tryptic Agar (CTA) is a microbiological growth medium used for various purposes, including the cultivation and identification of fastidious microorganisms, fermentation testing, and antimicrobial susceptibility testing. Here are the ingredients, materials, and composition typically used in CTA.
Ingredients:
- Cystine: Cystine is a sulfur-containing amino acid that provides essential nutrients for the growth of many bacteria, especially fastidious ones.
- Casein Peptone: Casein peptone is a protein digest that supplies a variety of nutrients, including amino acids, vitamins, and minerals, necessary for bacterial growth.
- Sodium Chloride: Sodium chloride helps maintain the osmotic balance of the medium, ensuring proper water exchange between bacterial cells and the environment.
- Sodium Sulfite: Sodium sulfite inhibits the growth of bacteria that produce hydrogen sulfide by converting hydrogen sulfide to less toxic thiosulfate.
- Phenol Red: Phenol red is a pH indicator dye that changes color in response to changes in pH. It shifts from red-pink to yellow as the medium becomes more acidic.
- Specific Carbohydrate (optional): CTA can be supplemented with a specific carbohydrate, depending on the intended use. This carbohydrate serves as a substrate for fermentation testing.
- Agar: Agar is added to the medium to give it a semisolid consistency, which is ideal for testing bacterial motility and performing fermentation tests.
Materials:
- Sterile Petri dishes or tubes: These are used to pour and solidify the CTA medium.
- Sterile inoculating loops or needles: These are used to inoculate the medium with bacteria.
- Incubator: An incubator is used to maintain the appropriate temperature for bacterial growth, typically around 35-37°C for most clinical applications.
- pH meter or pH indicator paper: These are used to confirm the pH of the medium before and after use.
Composition of Cystine Tryptic Agar:
| Ingredient | Quantity (per liter) | Purpose |
|---|---|---|
| Cystine | 0.5 grams | Essential nutrient for bacterial growth |
| Casein Peptone | 1.0 grams | Provides amino acids, vitamins, and minerals |
| Sodium Chloride | 0.5 grams | Maintains osmotic balance |
| Sodium Sulfite | 0.1 grams | Inhibits growth of hydrogen sulfide-producing bacteria |
| Phenol Red | 0.02 grams | pH indicator (changes color with pH) |
| Agar | 15-20 grams | Gives the medium a semisolid consistency for motility testing and solidification |
| Specific Carbohydrate (if added) | Varies | Serves as a substrate for fermentation testing |
Preparation of Cystine Tryptic Agar:
The preparation of Cystine Tryptic Agar (CTA) involves several steps to ensure the medium is properly formulated, sterilized, and ready for use in microbiological applications. Here’s a general overview of how to prepare CTA:
Materials and Ingredients Needed:
- Cystine Tryptic Agar powder (available commercially or prepared in-house).
- Distilled water or deionized water.
- Sterile Petri dishes, tubes, or containers for pouring the medium.
- Autoclave or pressure cooker for sterilization.
- pH meter or pH indicator paper for pH adjustment.
- Bunsen burner or alcohol lamp for aseptic technique.
- Weighing balance.
- Stirring rod or magnetic stirrer (optional).
Procedure:
- Weighing Ingredients:
- Weigh the appropriate quantity of Cystine Tryptic Agar powder according to the manufacturer’s instructions or your laboratory’s specific formulation. The quantities mentioned earlier in this conversation can serve as general guidelines.
- Mixing with Water:
- In a clean and sterile container, add the weighed CTA powder to a measured volume of distilled or deionized water. The amount of water will depend on the manufacturer’s instructions or your intended batch size. Typically, it’s about 1 liter of water per batch.
- Stirring (Optional):
- If necessary, use a stirring rod or magnetic stirrer to ensure the agar powder is thoroughly mixed with the water. Proper mixing is essential to prevent clumps or uneven distribution of ingredients.
- pH Adjustment:
- Measure the pH of the mixture using a pH meter or pH indicator paper. The pH should be adjusted to the desired level, typically around pH 7.4, which is close to neutral. You can adjust the pH by adding small amounts of acid (e.g., hydrochloric acid) or base (e.g., sodium hydroxide) as needed.
- Sterilization:
- Pour the prepared CTA medium into sterile containers, such as Petri dishes or tubes, ensuring they are properly labeled.
- Autoclave the containers at the appropriate temperature and pressure for sterilization. Typically, this involves autoclaving at 121°C (250°F) at 15 psi for 15-20 minutes.
- Cooling and Solidification:
- After sterilization, allow the CTA medium to cool down but not solidify completely. It should be in a liquid state, but still warm.
- Inoculation (Optional):
- If you need to inoculate the CTA medium with specific microorganisms for testing, do so while the medium is still in a liquid state. Use aseptic technique to avoid contamination.
- Solidification:
- Pour the warm, sterilized CTA medium into Petri dishes or containers and allow it to solidify at room temperature.
- Storage:
- Store the prepared CTA plates or tubes in a cool, dry place, protecting them from light if necessary.
Required Specimins for Culturing on CTA:
- Urethral Swabs: Used for the isolation of Neisseria gonorrhoeae, the causative agent of gonorrhea, a sexually transmitted infection.
- Cervical Swabs: Similar to urethral swabs, cervical swabs can be used to isolate Neisseria gonorrhoeae from female patients.
- Cerebrospinal Fluid (CSF): CTA can be used to detect Neisseria meningitidis in CSF samples from patients with suspected meningitis, a serious infection of the meninges surrounding the brain and spinal cord.
- Throat Swabs: Throat swabs can be used to isolate various microorganisms, including Streptococcus pneumoniae, a common cause of respiratory infections.
- Sputum Samples: For the isolation and identification of bacteria causing respiratory infections, such as Haemophilus influenzae.
- Genital Swabs: Swabs from genital lesions or wounds may be cultured on CTA to isolate and identify specific pathogens.
- Wound Swabs: Used for the isolation and identification of bacteria causing wound infections.
- Blood Cultures: Blood cultures can be processed on CTA to identify fastidious bacteria that may be causing septicemia.
- Clinical Specimens with Suspected Fastidious Pathogens: If there is clinical suspicion of infections caused by fastidious microorganisms like Neisseria spp., Streptococcus pneumoniae, or Haemophilus influenzae, specimens from the relevant sites can be cultured on CTA.
Usage Procedure of CTA:
The usage procedure of Cystine Tryptic Agar (CTA) involves the preparation of the medium and its application for various microbiological tests, including the isolation of fastidious microorganisms, detection of motility, fermentation tests, and antimicrobial susceptibility testing. Here’s a step-by-step guide on how to use CTA:
1. Preparation of CTA:
- Prepare the CTA medium as per the previous instructions, including the weighing of ingredients, mixing, pH adjustment, sterilization, cooling, and pouring into sterile containers (e.g., Petri dishes or tubes).
2. Inoculation:
- Depending on your specific testing objectives, inoculate the CTA medium with the appropriate specimen or microorganism of interest. Use aseptic technique to prevent contamination.
3. Isolation and Cultivation of Fastidious Microorganisms:
- If your goal is to isolate and cultivate fastidious microorganisms, inoculate the CTA medium with a specimen (e.g., swab or clinical sample) suspected of containing the target microbe.
- Incubate the inoculated CTA plates or tubes at the appropriate temperature for the microorganism being sought (e.g., 35-37°C for most clinical specimens).
- Observe the plates daily for growth and colony formation, which can be used for further identification and testing.
4. Detection of Bacterial Motility:
- For detecting bacterial motility, perform a stab inoculation by inserting a sterile inoculating needle or loop into the CTA medium and withdrawing it in a straight line.
- Motile bacteria will spread out from the stab line, creating turbidity or cloudiness throughout the medium.
- Non-motile bacteria will grow only along the stab line and leave the surrounding medium clear.
5. Performance of Fermentation Tests:
- To perform fermentation tests, supplement the CTA medium with a specific carbohydrate that matches your testing objectives.
- Inoculate the medium with the microorganism being tested.
- Incubate the medium at the appropriate temperature for the microorganism.
- Observe for color changes in the medium. A change from red to yellow indicates carbohydrate fermentation and acid production by the microorganism.
6. Antimicrobial Susceptibility Testing:
- For antimicrobial susceptibility testing, you can use CTA plates with antibiotic-impregnated discs.
- Place the discs on the surface of the CTA medium, ensuring they are evenly distributed.
- Incubate the plates at the appropriate temperature for the microorganism and antibiotic being tested.
- Observe for zones of inhibition around the antibiotic discs. A lack of growth around a disc indicates susceptibility to that antibiotic.
7. Interpretation:
- Interpret the results based on your specific testing objectives. For example, in antimicrobial susceptibility testing, the size of the inhibition zone can be used to determine the microorganism’s susceptibility or resistance to the tested antibiotics.
Result Interpretation of Cystine Tryptic Agar:
The results of Cystine Tryptic Agar (CTA) are interpreted based on the following:
- Growth: If growth is observed on the CTA plate, the bacterium is able to grow in the medium. This indicates that the bacterium is not a fastidious microorganism.
- Motility: If the bacterium is motile, it will spread out from the stab line on the CTA plate.
- Fermentation: If the bacterium can ferment the carbohydrate in the CTA medium, it will produce acid, which will cause the phenol red pH indicator in the medium to change color from red to yellow.
| Bacterium | Glucose | Sucrose | Maltose |
|---|---|---|---|
| Neisseria gonorrhoeae | + | – | – |
| Neisseria meningitidis | + | + | – |
| Streptococcus pneumoniae | + | + | – |
| Haemophilus influenzae | + | – | – |
| Escherichia coli | + | + | + |
| Salmonella spp. | + | + | + |
| Shigella spp. | + | + | + |
| Pseudomonas aeruginosa | – | + | + |
| Acinetobacter baumannii | – | + | + |
| Stenotrophomonas maltophilia | + | + | + |
| Streptococcus pyogenes | + | + | + |
| Staphylococcus aureus | + | + | + |
| Enterococcus faecalis | + | + | + |
Coloney Characteristics of Neisseria Spp:
The colony characteristics of Neisseria spp. can be assessed through various tests, including fermentation tests and motility tests, as described. Here’s a summary of the colony characteristics based on these tests:
Fermentation Test:
- Positive Reaction: The development of a yellow color change in the inoculated area (stab line) of the medium indicates that the carbohydrate has been fermented, resulting in acid production.
- Negative Reaction: A red-pink or deep red color in the medium suggests that the carbohydrate has not been utilized for fermentation, and peptone degradation has occurred instead. This typically results in a deeper red to orange color in the medium.
Motility Test:
- Positive Test: A positive motility test is indicated by turbidity or cloudy growth extending from the line of inoculation. This suggests that the bacteria are motile and can move away from the point of inoculation.
- Negative Test: A negative motility test is characterized by growth only along the stab line, leaving the surrounding medium clear. This indicates that the bacteria are non-motile and do not spread throughout the medium.
These tests can provide valuable information for the identification and characterization of Neisseria species, particularly in a clinical microbiology laboratory setting. It’s important to note that Neisseria species can exhibit variations in their ability to ferment specific carbohydrates and in their motility, so these tests are used in conjunction with other biochemical and molecular methods for accurate species identification.
Growth of Other Bacterias on Cystine Tryptic Agar:
Cystine Tryptic Agar (CTA) is a semisolid growth medium that is used for the isolation and cultivation of fastidious microorganisms, such as Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. However, a variety of other bacteria can also grow on CTA.
Some common examples of other bacteria that can grow on CTA include:
- Enterobacteriaceae: Enterobacteriaceae are a large family of Gram-negative bacteria that includes many common pathogens, such as Escherichia coli, Salmonella spp., and Shigella spp. Enterobacteriaceae typically grow on CTA as small, round, translucent colonies.
- Non-fermenting Gram-negative bacteria: Non-fermenting Gram-negative bacteria are a diverse group of bacteria that do not ferment lactose. Some common examples of non-fermenting Gram-negative bacteria that can grow on CTA include Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. Non-fermenting Gram-negative bacteria typically grow on CTA as small, round, opaque colonies.
- Gram-positive cocci: Gram-positive cocci are a large group of bacteria that includes many common pathogens, such as Streptococcus pyogenes, Staphylococcus aureus, and Enterococcus faecalis. Gram-positive cocci typically grow on CTA as small, round, opaque colonies.
Limitations of of CTA:
- Incomplete Identification: CTA is a selective medium that provides preliminary information about the fermentation capabilities of microorganisms. It may not provide complete identification, so further testing (biochemical, immunological, molecular, or mass spectrometry) is often required for accurate species identification.
- Limited Acid Production: Neisseria species may produce only small amounts of acid during carbohydrate utilization, which can lead to weak acid reactions. This can make interpretation of test results challenging.
- pH Indicator Changes: Prolonged incubation can lead to changes in the pH indicator, potentially affecting the interpretation of results. It’s important to read results within the recommended time frame.
- Aerobic Incubation Required: CTA should be incubated aerobically, not in a CO2-enriched environment. Incubation in CO2 may lead to erroneous results.
- Heavy Inoculum Needed: CTA requires a substantial inoculum for reliable results. An insufficient inoculum may lead to inaccurate test outcomes.
- Improper Inoculation: Inoculating the medium improperly or not reaching the bottom of the tube can result in weak acid reactions, making it difficult to interpret test results.
- Alkaline By-products: Peptone utilization by microorganisms can produce alkaline by-products. Prolonged incubation may lead to a reversion reaction, where these alkaline by-products mask the acid by-products from carbohydrate utilization, affecting test interpretation.
- Maltose Utilization: Some strains of meningococci, particularly sulfonamide-resistant strains, may not produce acid from maltose. This may require repeated subcultures to non-inhibitory media to restore their maltose-utilizing capability.
- Inability to Grow Certain Strains: Some strains of gonococci may require additional compounds not provided by CTA medium formulations. As a result, they may not grow on CTA media.
- Lack of Differentiation: CTA is primarily used for fermentation testing and preliminary characterization. It may not provide the level of differentiation required for all clinical or research purposes.
Home | Blog | About Us | Contact Us | Disclaimer
Possible References Used