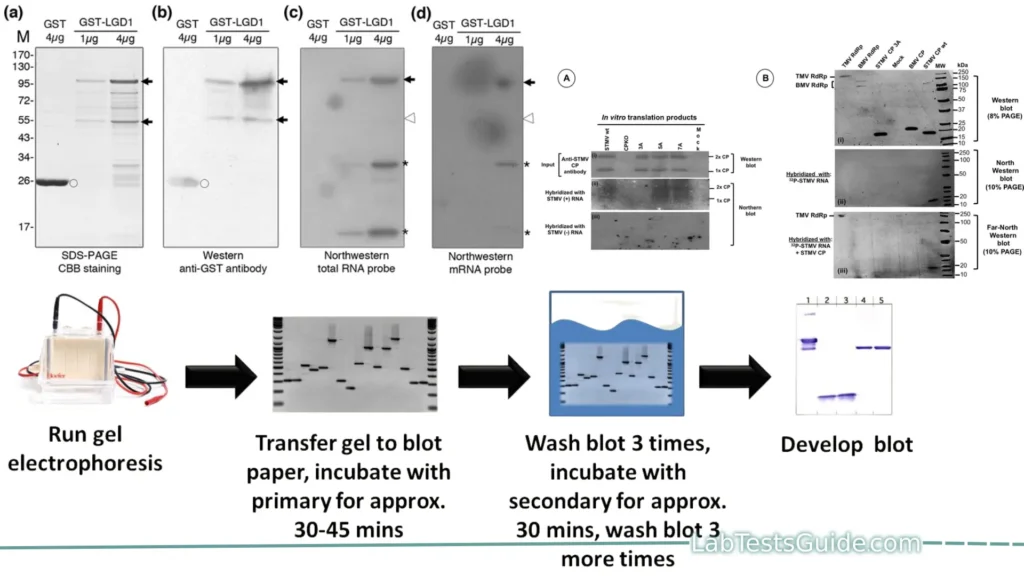
Northwestern blotting, also known as the northwestern assay, is a hybrid analytical technique of the Western Blot and the Northern Blot, and is used in molecular biology to detect interactions between RNA and proteins.
Key points of Northwestern Blotting:
Northwestern blotting is a specialized technique used to study RNA-protein interactions. Here are 18 key points to understand about Northwestern blotting:
- Purpose: Northwestern blotting is used to identify and characterize RNA-protein interactions, helping researchers understand the roles of specific RNA molecules in various biological processes.
- RNA Probe: A labeled RNA molecule is used as a probe to detect RNA-protein interactions. The RNA probe is usually single-stranded and may be synthesized in vitro or isolated from cells.
- Protein Extraction: Proteins are extracted from biological samples (e.g., cells or tissues) of interest. These proteins may include the target protein suspected to interact with the RNA.
- Native Gel Electrophoresis: Proteins are separated by native gel electrophoresis, maintaining their native structure. Separation is based on charge and size, allowing proteins to retain their RNA-binding activity.
- Protein Transfer: After electrophoresis, proteins are transferred from the gel to a membrane, typically using a process similar to Western blotting.
- Membrane Blocking: The membrane is blocked to prevent non-specific binding of the RNA probe.
- Renaturation: Extracted proteins on the membrane are renatured to allow them to regain their native conformation.
- RNA-Protein Binding Assay: The labeled RNA probe is applied to the membrane containing renatured proteins. If RNA-protein interactions occur, the RNA probe will bind to the target protein.
- Detection Methods: Detection can be achieved using autoradiography (for radioactive probes) or by using antibodies specific to the label on the RNA probe (e.g., anti-biotin or anti-digoxigenin antibodies for non-radioactive probes).
- Visualization: The presence of RNA-protein complexes is visualized as bands or spots on the membrane.
- Controls: Positive and negative controls are used to validate the specificity of RNA-protein interactions.
- 12. RNA-Protein Interactome: Northwestern blotting can provide information about the RNA-binding proteins in a sample, contributing to the study of the RNA-protein interactome.
- RNA-Protein Complex Stability: Researchers can assess the stability of RNA-protein complexes by varying experimental conditions, such as salt concentrations or temperature.
- RNA Binding Site Identification: Northwestern blotting can help identify the specific regions of an RNA molecule that interact with a protein.
- Comparative Studies: Researchers can compare RNA-protein interactions between different samples or conditions to gain insights into regulatory mechanisms.
- Crosslinking: In some cases, crosslinking agents may be used to stabilize RNA-protein interactions before performing Northwestern blotting.
- Applications: Northwestern blotting is applied in various fields, including molecular biology, genetics, and biochemistry, to investigate RNA-protein interactions in processes such as transcriptional regulation, RNA processing, and RNA localization.
- Complementary Techniques: Northwestern blotting is often complemented by other techniques like RNA immunoprecipitation (RIP) and electrophoretic mobility shift assay (EMSA) to further validate and characterize RNA-protein interactions.
Defination of Northwestern Blotting:
Northwestern blotting is a laboratory technique used to study RNA-protein interactions by detecting and characterizing the binding of RNA molecules to specific proteins.
Background and Significance:
History and Background:
- Development of Blotting Techniques: Northwestern blotting evolved from Southern blotting (for DNA) and Western blotting (for proteins), both of which were developed in the 1970s.
- Southern Blotting (1975): Enabled the detection of specific DNA sequences through DNA probe hybridization.
- Western Blotting (1979): Extended the concept to protein detection through antibody-based techniques.
- Northwestern Blotting (1990s): Developed to study RNA-protein interactions, with “Northwestern” as an analogy to “Western” and “Southern” blotting.
Significance:
- Studying RNA-Protein Interactions: Northwestern blotting is a crucial tool for investigating how RNA molecules interact with specific proteins in cells.
- Understanding Regulatory Mechanisms: Helps unravel the regulatory mechanisms governing gene expression, RNA processing, and cellular functions.
- Disease Research: Used in studying RNA-protein interactions associated with diseases like cancer, neurodegenerative disorders, and viral infections, aiding in disease understanding and potential therapies.
- Drug Discovery: Essential for identifying drug targets and designing therapeutic interventions by elucidating RNA-protein interactions.
- Complementing Techniques: Often used alongside other methods like RNA immunoprecipitation (RIP) and electrophoretic mobility shift assay (EMSA) to provide a comprehensive view of RNA-protein interactions.
Purpose of Northwestern Blotting:
- Studying RNA-Protein Interactions: Northwestern blotting is primarily used to investigate and characterize interactions between RNA molecules and specific proteins.
- Identifying RNA-Binding Proteins: It helps identify and verify proteins that have a binding affinity for particular RNA sequences or structures.
- Understanding Gene Regulation: Provides insights into the regulatory mechanisms of gene expression, including post-transcriptional regulation.
- RNA Processing Analysis: Facilitates the study of RNA processing events, such as splicing and cleavage, by identifying associated proteins.
- RNA Localization Studies: Aids in understanding how RNA molecules are localized within cells and how this process is mediated by RNA-protein interactions.
- Disease Mechanisms: Helps uncover RNA-protein interactions involved in diseases, contributing to the understanding of disease mechanisms.
- Drug Target Discovery: Identifies potential drug targets by revealing critical RNA-protein interactions that can be manipulated for therapeutic purposes.
- Comparative Analysis: Allows for the comparison of RNA-protein interactions under different experimental conditions or in various cell types.
- Functional Genomics: Supports functional genomics research by elucidating the roles of specific RNA molecules and their binding partners.
- Cellular Signaling: Offers insights into how RNA-protein interactions contribute to cellular signaling pathways and responses.
- Biological Pathway Investigation: Helps uncover the involvement of RNA-protein interactions in biological pathways, such as RNA transport or translation.
- Biomarker Discovery: Can lead to the identification of RNA-protein interaction biomarkers for diagnostic or prognostic purposes.
- Viral Replication Studies: Used to understand how RNA viruses interact with host cell proteins during replication.
- RNA Structure-Function Relationship: Investigates how RNA secondary and tertiary structures influence their interactions with specific proteins.
- Ribonucleoprotein Complex Analysis: Aids in the characterization of ribonucleoprotein complexes that play critical roles in cellular processes.
- RNA Editing Investigations: Studies the involvement of RNA-binding proteins in RNA editing events.
- RNA Stability and Turnover: Helps assess how RNA-protein interactions influence RNA stability and degradation.
- Cellular Localization Signals: Identifies signals within RNA molecules that determine their cellular localization based on protein interactions.
Applications of Northwestern Blotting:
- RNA-Protein Interaction Analysis: The primary application is to study and characterize RNA-protein interactions, providing insights into the binding partners of specific RNA molecules.
- Gene Regulation Studies: It helps unravel the mechanisms of post-transcriptional gene regulation by identifying proteins involved in mRNA stability, translation, and processing.
- RNA Processing Investigations: Used to examine RNA splicing, cleavage, and modifications by identifying proteins that participate in these processes.
- RNA Localization Research: Explores how RNA molecules are transported and localized within cells, uncovering RNA-protein interactions responsible for subcellular distribution.
- Disease Mechanism Exploration: Applied to understand RNA-protein interactions implicated in diseases, including cancer, neurodegenerative disorders, and viral infections.
- Drug Target Discovery: Identifies potential therapeutic targets by revealing critical RNA-protein interactions that can be manipulated for drug development.
- Comparative Analysis: Enables the comparison of RNA-protein interactions under different conditions or in various cell types, aiding in understanding cellular responses.
- Functional Genomics: Supports functional genomics research by elucidating the roles of specific RNA molecules and their binding partners in cellular processes.
- Cell Signaling Investigations: Provides insights into how RNA-protein interactions contribute to cellular signaling pathways and responses.
- Biological Pathway Exploration: Helps uncover the involvement of RNA-protein interactions in biological pathways, such as RNA transport, ribosome assembly, and translation.
- Biomarker Discovery: Can lead to the identification of RNA-protein interaction biomarkers for diagnostic or prognostic purposes in various diseases.
- Viral Replication Studies: Used to understand how RNA viruses interact with host cell proteins during the replication cycle.
- RNA Structure-Function Relationship: Investigates how RNA secondary and tertiary structures influence their interactions with specific proteins.
- Ribonucleoprotein Complex Characterization: Aids in the comprehensive characterization of ribonucleoprotein complexes that play crucial roles in cellular processes.
- RNA Editing Analysis: Studies the involvement of RNA-binding proteins in RNA editing events, such as A-to-I editing.
- RNA Stability and Turnover Assessment: Helps assess how RNA-protein interactions influence RNA stability and degradation processes.
- Cellular Localization Signals Identification: Identifies signals within RNA molecules that determine their cellular localization based on interactions with specific proteins.
- RNA Transport Mechanism Exploration: Investigates the molecular mechanisms of RNA transport within cells, identifying proteins involved in this process.
Principles of Northwestern Blotting:
- RNA-Protein Interaction Detection: Northwestern blotting is designed to detect and characterize interactions between RNA molecules and specific proteins in a biological sample.
- RNA Probe Usage: A labeled RNA probe, typically single-stranded and marked with a radioactive or non-radioactive label, is used as a tool to identify RNA-protein complexes.
- Protein Extraction: Proteins are extracted from the sample under investigation, which may include the target protein suspected to interact with the RNA of interest.
- Native Gel Electrophoresis: Extracted proteins are separated using native gel electrophoresis, a technique that maintains the proteins’ native conformation based on charge and size.
- Protein Transfer to Membrane: Following electrophoresis, proteins are transferred onto a membrane (e.g., nitrocellulose or PVDF), similar to Western blotting.
- Membrane Blocking: The membrane is blocked to prevent non-specific binding of the RNA probe.
- Renaturation: Proteins on the membrane are renatured to allow them to regain their native conformation, which is crucial for RNA binding.
- RNA-Protein Interaction Assay: The labeled RNA probe is applied to the membrane, and if RNA-protein interactions occur, the RNA probe will specifically bind to the target proteins.
- Detection Methods: The presence of RNA-protein complexes is detected either through autoradiography (for radioactive labels) or by using antibodies specific to the label (e.g., anti-biotin or anti-digoxigenin antibodies for non-radioactive labels).
- Visualization: RNA-protein complexes are visualized as bands or spots on the membrane, indicating which proteins interact with the labeled RNA.
- Control Experiments: Positive and negative controls are often included to validate the specificity of RNA-protein interactions detected.
- RNA-Protein Interactome: Northwestern blotting can provide insights into the RNA-binding proteins in a sample, contributing to the study of the RNA-protein interactome.
- Complex Stability Assessment: Researchers can assess the stability of RNA-protein complexes under various experimental conditions, such as altering salt concentrations or temperature.
- RNA Binding Site Identification: The technique can help identify the specific regions of an RNA molecule that interact with a protein.
- Comparative Studies: Northwestern blotting allows for comparisons of RNA-protein interactions between different samples or conditions, aiding in understanding regulatory mechanisms.
- Crosslinking Option: In some cases, crosslinking agents may be used to stabilize RNA-protein interactions before performing Northwestern blotting.
- Complementary Techniques: Northwestern blotting is often used alongside other methods like RNA immunoprecipitation (RIP) and electrophoretic mobility shift assay (EMSA) to validate and characterize RNA-protein interactions.
Procedure for Northwestern Blotting:
- RNA Isolation: Isolate RNA of interest, which can be synthesized in vitro or obtained from biological samples. Ensure RNA purity and integrity.
- RNA Denaturation and Labeling: Denature the RNA, often by heating, and label it with a radioactive or non-radioactive marker (e.g., biotin or digoxigenin).
- Protein Extraction: Extract proteins from the biological sample under study, which may include the target protein expected to interact with the RNA.
- Native Gel Electrophoresis: Separate proteins by native gel electrophoresis, maintaining their native conformation. Proteins are separated based on charge and size.
- Protein Transfer: Transfer the separated proteins from the gel to a membrane (typically nitrocellulose or PVDF) using a transfer apparatus.
- Membrane Blocking: Block the membrane to prevent non-specific binding by incubating it in a blocking solution (e.g., milk or BSA).
- Renaturation: To allow proteins to regain their native conformation, renature the membrane by incubating it under appropriate conditions.
- RNA-Protein Interaction Assay: Apply the labeled RNA probe (from step 2) to the membrane containing renatured proteins. Incubate to allow RNA-protein binding.
- Washing Steps: Wash the membrane to remove unbound RNA probe, maintaining specificity.
- Detection Methods: Detect RNA-protein complexes either by autoradiography (for radioactive labels) or by using specific antibodies against the label (e.g., anti-biotin or anti-digoxigenin antibodies for non-radioactive labels).
- Visualization: Visualize the presence of RNA-protein complexes as bands or spots on the membrane.
- Controls: Include positive and negative controls to validate the specificity of RNA-protein interactions detected.
- Data Analysis: Analyze the results, including the identification of interacting proteins and their relationship to the labeled RNA probe.
- Complex Stability Assessment: Optionally, assess the stability of RNA-protein complexes under different conditions, e.g., varying salt concentrations or temperature.
- RNA Binding Site Identification: If needed, perform additional experiments to identify specific RNA binding sites on the target proteins.
- Comparative Studies: Compare RNA-protein interactions between different samples or experimental conditions to gain insights into regulatory mechanisms.
- Data Interpretation: Interpret the data in the context of the biological question or hypothesis being investigated.
- Documentation: Document the experimental procedures, results, and conclusions for future reference and reporting.
Northwestern blotting is a stepwise procedure used to investigate RNA-protein interactions, providing valuable insights into cellular processes, gene regulation, and disease mechanisms.
Materials and Reagents:
- RNA Sample: Isolated RNA of interest, which can be synthesized in vitro or obtained from biological samples.
- RNA Label: A labeled RNA probe, typically marked with a radioactive or non-radioactive label (e.g., biotin or digoxigenin), used to detect RNA-protein interactions.
- Protein Extraction Buffer: A buffer solution containing detergents and protease inhibitors to extract proteins from the sample.
- Native Gel Electrophoresis Reagents: Including acrylamide, bis-acrylamide, Tris-HCl, ammonium persulfate (APS), and tetramethylethylenediamine (TEMED) for gel preparation.
- Electrophoresis Apparatus: Gel electrophoresis chamber and power supply for native gel electrophoresis.
- Transfer Membrane: Nitrocellulose or PVDF membrane for protein transfer from the gel.
- Transfer Buffer: Buffer solution (e.g., Towbin buffer) for protein transfer from the gel to the membrane.
- Blocking Solution: Typically a solution containing milk (e.g., skim milk or bovine serum albumin) to block non-specific protein binding on the membrane.
- RNA-Protein Interaction Buffer: A buffer that maintains RNA-protein interactions during the assay (e.g., binding buffer containing salts and detergents).
- RNA-Protein Interaction Assay Controls: Positive and negative control samples to validate the specificity of RNA-protein interactions detected.
- RNA-Protein Interaction Detection Reagents:
- For radioactive labels: Autoradiography film or phosphorimager screens.
- For non-radioactive labels: Antibodies specific to the label (e.g., anti-biotin or anti-digoxigenin antibodies) conjugated to enzymes (e.g., horseradish peroxidase or alkaline phosphatase) for chemiluminescent or colorimetric detection.
- Washing Buffers: Buffers (e.g., Tris-buffered saline with Tween-20, TBST) for washing the membrane to remove unbound RNA probe.
- Blocking Reagent: Used to minimize non-specific binding of detection antibodies in non-radioactive labeling systems.
- Substrate for Enzyme Detection: Substrates like chemiluminescent or colorimetric substrates for enzymatic detection methods.
- Protein Size Markers: Molecular weight markers for estimating protein sizes during gel electrophoresis.
- RNA Markers: Molecular weight markers for estimating RNA sizes during gel electrophoresis.
- Protein Stain: Coomassie Brilliant Blue or other protein stains for visualizing proteins on the gel before transfer.
- RNA Stain: Ethidium bromide or other RNA stains for visualizing RNA on the gel before transfer.
- Gel Documentation System: A system for capturing images of gels and blots.
- Laboratory Supplies: Pipettes, microcentrifuge tubes, electrophoresis chambers, transfer apparatus, and other standard lab equipment.
- Safety Gear: Appropriate personal protective equipment (PPE) such as lab coats, gloves, and safety goggles.
Step-by-Step Protocol:
1. RNA Isolation and Labeling:
- Isolate RNA of interest from cells or tissues.
- Denature the RNA, and label it with a radioactive or non-radioactive marker (e.g., biotin or digoxigenin).
2. Protein Extraction:
- Extract proteins from the same cells or tissues using a suitable protein extraction buffer.
3. Native Gel Electrophoresis:
- Prepare a native polyacrylamide gel containing proteins.
- Load the protein samples onto the gel.
- Perform native gel electrophoresis to separate proteins based on charge and size.
4. Protein Transfer:
- Transfer proteins from the gel to a nitrocellulose or PVDF membrane using a transfer buffer and electrophoretic transfer apparatus.
5. Membrane Blocking:
- Block the membrane using a blocking solution (e.g., milk) to prevent non-specific binding.
6. Renaturation:
- Incubate the membrane to allow proteins to regain their native conformation.
7. RNA-Protein Interaction Assay:
- Apply the labeled RNA probe (from step 1) to the membrane.
- Incubate the membrane to allow RNA-protein binding.
8. Washing Steps:
- Wash the membrane to remove unbound RNA probe, maintaining specificity.
9. Detection Methods:
- For radioactive labels: Expose the membrane to autoradiography film or phosphorimager screens.
- For non-radioactive labels: Incubate the membrane with detection antibodies specific to the label, followed by substrate addition for chemiluminescent or colorimetric detection.
10. Visualization:
- Visualize the presence of RNA-protein complexes as bands or spots on the membrane.
11. Controls:
- Include positive and negative control samples to validate the specificity of RNA-protein interactions detected.
12. Data Analysis:
- Analyze the results, including identifying interacting proteins and their relationship to the labeled RNA.
13. Complex Stability Assessment (Optional):
- Assess the stability of RNA-protein complexes under different conditions, if needed.
14. RNA Binding Site Identification (Optional):
- Perform additional experiments to identify specific RNA binding sites on the target proteins.
15. Comparative Studies (Optional):
- Compare RNA-protein interactions between different samples or experimental conditions.
16. Data Interpretation:
- Interpret the data in the context of the biological question or hypothesis being investigated.
Result Interpretation:
- Presence of Bands or Spots: Presence of bands or spots on the membrane indicates the formation of RNA-protein complexes.
- Band Intensity: The intensity of bands can provide information about the strength of RNA-protein interactions. Stronger bands suggest tighter binding.
- Protein Identity: Identify the proteins interacting with the labeled RNA probe based on their molecular weights, which can be estimated using molecular weight markers.
- Multiple Bands: Multiple bands may indicate multiple RNA-protein complexes or protein isoforms.
- Specificity: Ensure specificity by comparing results with positive and negative controls. Specific interactions should be present in the sample of interest but not in the negative control.
- Shifted Bands: Shifted bands on the gel compared to controls may suggest changes in RNA-protein interactions under different conditions.
- Comparative Analysis: Compare results between different samples or conditions to understand variations in RNA-protein interactions.
- Complex Stability: Assess the impact of varying experimental conditions (e.g., salt concentrations or temperature) on complex stability if stability assessments were performed.
- RNA Binding Sites: If RNA binding site identification was conducted, analyze the specific regions of RNA that interact with the proteins.
- Qualitative vs. Quantitative Analysis: Depending on the goals, perform qualitative or quantitative analysis of RNA-protein interactions. Quantitative analysis may involve densitometry to measure band intensities.
- Correlation with Biological Processes: Interpret results in the context of the biological question or hypothesis being investigated. How do the identified RNA-protein interactions relate to cellular functions or disease mechanisms?
- Validation: Validate results through complementary experiments or techniques such as RNA immunoprecipitation (RIP) or electrophoretic mobility shift assay (EMSA).
- Publication and Reporting: Prepare figures and document the interpretation of results clearly in research publications or reports.
- Further Studies: Based on the findings, plan further studies to explore the functional significance of the identified RNA-protein interactions or their role in specific cellular processes.
- Biological Significance: Assess the biological significance of the identified interactions and consider how they contribute to gene regulation, RNA processing, or other cellular functions.
Troubleshooting and Tips:
- Low Signal Intensity: If bands are faint or absent, check the efficiency of labeling the RNA probe or the efficiency of protein transfer to the membrane.
- High Background: Excessive background staining can result from inadequate blocking or insufficient washing steps. Ensure thorough blocking and washing.
- Non-Specific Bands: Non-specific bands may appear due to cross-reactivity of antibodies or non-specific binding of the RNA probe. Use appropriate controls to confirm specificity.
- Uneven Gel Loading: Ensure even protein loading on the gel to prevent skewed results. Use molecular weight markers for reference.
- Protein Aggregation: Protein aggregates may lead to unexpected results. Optimize protein extraction and handling procedures.
- Incomplete Transfer: Check for complete protein transfer from the gel to the membrane. Confirm transfer efficiency using Ponceau S staining or reversible protein staining.
- Inadequate Renaturation: If the proteins fail to renature properly on the membrane, adjust renaturation conditions (e.g., incubation time or temperature).
- Contamination: Ensure a clean working environment and use clean labware to prevent contamination.
- Insufficient RNA Probe Concentration: If RNA-protein interactions are weak, consider increasing the concentration of the RNA probe.
- RNA Probe Degradation: Use freshly prepared RNA probes to prevent degradation, especially for radioactive probes.
- Control Validation: Always include positive and negative controls to validate the specificity of interactions.
- Experimental Reproducibility: Standardize protocols and conditions for reproducible results.
Tips:
- Document all steps and conditions meticulously for troubleshooting purposes.
- Perform a pilot experiment to optimize conditions before conducting the actual Northwestern blot.
- Use high-quality reagents and perform quality control checks.
- Be patient and thorough during blocking, washing, and incubation steps.
- Seek guidance from experienced colleagues or mentors when encountering issues.
- Consider complementary techniques like RNA immunoprecipitation (RIP) or electrophoretic mobility shift assay (EMSA) for validation.
- Stay up-to-date with literature and advances in Northwestern blotting techniques for improved results.
Advantages and Disadvantages of Northwestern Blotting:
Advantages of Northwestern Blotting:
- Study RNA-Protein Interactions: Northwestern blotting is specifically designed to study RNA-protein interactions, providing insights into the roles of RNA molecules in cellular processes.
- Target Protein Identification: It helps identify and characterize proteins that bind to specific RNA sequences or structures.
- Understanding Regulatory Mechanisms: Offers a tool for unraveling post-transcriptional gene regulation and RNA processing mechanisms.
- Disease Research: Useful for investigating RNA-protein interactions involved in diseases, aiding in disease mechanism understanding and potential therapeutic target identification.
- Drug Discovery: Can identify potential drug targets by revealing critical RNA-protein interactions that can be manipulated for drug development.
- Comparative Analysis: Enables the comparison of RNA-protein interactions under different conditions or in various cell types.
- Functional Genomics: Supports functional genomics research by elucidating the roles of specific RNA molecules and their binding partners in cellular processes.
Disadvantages of Northwestern Blotting:
- Complexity: The technique can be technically challenging, requiring optimization of multiple steps for reliable results.
- Time-Consuming: The protocol involves several time-consuming steps, from RNA labeling to membrane probing and detection.
- Radioactive Materials: The use of radioactive probes involves safety considerations and regulatory compliance.
- Non-Specific Binding: Non-specific binding of the RNA probe or antibodies can lead to false-positive results.
- Protein Denaturation: Maintaining protein native conformation can be difficult, potentially affecting the accuracy of results.
- Low Throughput: Northwestern blotting is not suitable for high-throughput analysis and is typically performed for a limited number of samples.
- Expensive: The cost of reagents, antibodies, and specialized equipment can be high.
- Limited Quantitative Data: It provides qualitative or semi-quantitative data, making it less suitable for precise quantification of RNA-protein interactions.
- Complementary Techniques Required: Often, complementary techniques like RNA immunoprecipitation (RIP) are necessary to validate and provide more comprehensive insights.
Limitations of Northwestern Blotting:
- Complexity: The technique involves multiple steps and can be technically challenging, requiring optimization for reliable results.
- Time-Consuming: The protocol includes several time-consuming steps, from RNA labeling to membrane probing and detection.
- Radioactive Materials: The use of radioactive probes raises safety concerns and regulatory compliance issues.
- Non-Specific Binding: Non-specific binding of the RNA probe or antibodies can lead to false-positive results, requiring careful controls and optimization.
- Protein Denaturation: Maintaining native protein conformation during the procedure can be challenging, potentially affecting result accuracy.
- Low Throughput: Northwestern blotting is not suitable for high-throughput analysis and is typically performed for a limited number of samples.
- Expensive: The cost of reagents, antibodies, and specialized equipment can be prohibitive for some researchers.
- Limited Quantitative Data: The technique provides qualitative or semi-quantitative data, making it less suitable for precise quantification of RNA-protein interactions.
- Complementary Techniques Required: Often, complementary techniques like RNA immunoprecipitation (RIP) or electrophoretic mobility shift assay (EMSA) are necessary to validate and provide more comprehensive insights.
- Difficulty in Identifying RNA-Protein Binding Sites: Northwestern blotting does not directly provide information about the specific regions of RNA that interact with proteins.
- Sample Specificity: Results may be influenced by factors such as the choice of cell/tissue source and the specific RNA probe used.
- Incompatibility with Certain Proteins: Not all proteins may maintain their native conformation under the conditions used for Northwestern blotting, potentially leading to false-negative results.
- Sensitivity: Some RNA-protein interactions with weak affinities may not be detectable using this technique.
- Quantitative Challenges: Quantifying the degree of RNA-protein interaction can be challenging due to the absence of a linear relationship between signal intensity and concentration.
- Data Interpretation Complexity: Interpreting the results requires expertise, and band analysis can be subjective.
Variations and Modern Alternatives:
Variations of Northwestern Blotting:
- Far-Western Blotting: In this variation, instead of using labeled RNA probes, labeled purified proteins are used to probe a membrane containing immobilized RNA. It helps identify RNA-binding proteins.
- Southwestern Blotting: Similar to Northwestern blotting, but it is used to study DNA-protein interactions rather than RNA-protein interactions.
- Reverse Northwestern Blotting: In this reverse approach, proteins are labeled and probed against an immobilized library of RNA molecules to identify RNA-binding proteins.
- Chemiluminescent Detection: Traditional autoradiography can be replaced with chemiluminescent detection for increased sensitivity and safety.
Modern Alternatives to Northwestern Blotting:
- RNA Immunoprecipitation (RIP): RIP involves immunoprecipitating RNA-protein complexes using specific antibodies against the protein of interest, followed by RNA analysis. It offers greater specificity and sensitivity.
- Cross-Linking Immunoprecipitation (CLIP): CLIP combines UV cross-linking of RNA-protein complexes in vivo with immunoprecipitation, allowing the identification of in vivo RNA-protein interactions.
- RNA Sequencing (RNA-Seq): RNA-Seq provides a global view of RNA expression and can be used to identify RNA-binding proteins through methods like CLIP-Seq and PAR-CLIP.
- Electrophoretic Mobility Shift Assay (EMSA): EMSA is a technique that directly assesses the binding of RNA to proteins by measuring changes in their electrophoretic mobility in a gel.
- Proximity Ligation Assay (PLA): PLA detects interactions between proteins and RNA by utilizing proximity probes and fluorescence-based detection.
- RNA Pull-Down Assays: These assays use biotin-labeled RNA probes to pull down interacting proteins from cell lysates, followed by mass spectrometry or Western blotting for protein identification.
- RNA-Centric Approaches: Modern approaches focus on understanding RNA functions and interactions using tools like CRISPR-based RNA labeling and RNA interactome capture techniques.
- Proteomics: Mass spectrometry-based proteomics approaches can identify RNA-binding proteins by analyzing the protein composition of RNA-protein complexes.
- High-Throughput Techniques: Technologies like CLIP-Seq and PAR-CLIP-Seq enable the high-throughput profiling of RNA-protein interactions at a genomic scale.
Comparison of Northwestern Blotting with Modern Techniques:
| Aspect | Northwestern Blotting | Modern Techniques |
|---|---|---|
| Type of Interaction Studied | RNA-protein interactions | RNA-protein interactions and more |
| Detection Principle | Labeled RNA probe and autoradiography or antibody-based detection for non-radioactive labels | RNA immunoprecipitation (RIP), CLIP-Seq, RNA-Seq, EMSA, mass spectrometry, PLA, RNA pull-down assays, and more |
| Sensitivity | Moderate to high sensitivity | High sensitivity for most techniques |
| Specificity | Requires controls for specificity | Offers high specificity, especially in RIP and CLIP approaches |
| Quantitative Data | Semi-quantitative, challenging for precise quantification | Offers quantitative data in many cases, especially in RNA-Seq and mass spectrometry-based approaches |
| Throughput | Low to moderate throughput | Varies depending on the technique, but many are suitable for high throughput |
| Sample Requirement | Moderate sample requirements | Varies depending on the technique, some requiring smaller sample amounts |
| Speed of Analysis | Time-consuming | Varies but some modern techniques can provide faster results |
| Cost | Moderate cost for reagents and equipment | Cost varies widely depending on the technique and instrumentation |
| Ease of Use | Requires expertise and optimization | Some modern techniques may require less optimization and are more user-friendly |
| Safety Considerations | Involves radioactive materials (for radioactive labels) | Generally safer as many modern techniques do not involve radioactivity |
| Data Interpretation | Requires expertise in gel analysis | May offer more straightforward data analysis and visualization tools |
| Applications | Primarily for RNA-protein interactions | Versatile for a wide range of RNA-related studies, including RNA-protein interactions, RNA expression, and RNA function |
| Biological Insights | Offers insights into RNA-protein interactions but limited insight into other aspects of RNA biology | Offers a broader view of RNA biology, including transcriptome-wide analysis and identification of RNA-binding proteins |
FAQs:
1. What is Northwestern blotting?
- Northwestern blotting is a laboratory technique used to study RNA-protein interactions. It involves transferring RNA-protein complexes from a gel to a membrane and detecting the presence of specific RNA-binding proteins.
2. How does Northwestern blotting work?
- Northwestern blotting starts with isolating RNA and labeling it with a marker. Extracted proteins are separated on a gel, transferred to a membrane, and probed with the labeled RNA. The presence of RNA-protein complexes is detected using autoradiography or antibodies.
3. What is the significance of studying RNA-protein interactions?
- Understanding RNA-protein interactions is crucial for unraveling post-transcriptional gene regulation, RNA processing, disease mechanisms, and potential drug targets in various biological processes.
4. What are the advantages of Northwestern blotting?
- Northwestern blotting is specific for RNA-protein interactions, helps identify interacting proteins, and provides insights into gene regulation and disease mechanisms.
5. What are the limitations of Northwestern blotting?
- Some limitations include complexity, time consumption, safety concerns with radioactive labels, and the need for optimization. It also provides qualitative or semi-quantitative data.
6. Are there alternatives to Northwestern blotting for studying RNA-protein interactions?
- Yes, modern alternatives include RNA immunoprecipitation (RIP), CLIP-Seq, RNA-Seq, EMSA, mass spectrometry, PLA, RNA pull-down assays, and others, each with its own strengths and applications.
7. Can Northwestern blotting be used for high-throughput analysis?
- Northwestern blotting is typically not suitable for high-throughput analysis due to its time-consuming nature. Modern techniques like RIP and CLIP-Seq offer higher throughput options.
8. Is Northwestern blotting still widely used in research today?
- Northwestern blotting remains a valuable technique in specific contexts where its strengths align with research goals. However, researchers often choose from a range of modern alternatives based on their specific needs.
9. What precautions should be taken when using radioactive probes in Northwestern blotting?
- Safety measures include working in designated areas, using appropriate protective gear, and following regulations for handling and disposing of radioactive materials.
10. How can I validate the specificity of RNA-protein interactions detected using Northwestern blotting?
- Include positive and negative controls in your experiment to validate the specificity of the interactions. Positive controls should contain known interacting proteins, while negative controls should lack specific interactions.
Conclusion:
In conclusion, Northwestern blotting is a valuable laboratory technique designed for the study of RNA-protein interactions. This method plays a crucial role in molecular biology and biochemistry research, providing insights into post-transcriptional gene regulation, RNA processing, and disease mechanisms. By employing labeled RNA probes, gel electrophoresis, and membrane-based detection, researchers can identify and characterize RNA-binding proteins and their interactions with specific RNA molecules.
While Northwestern blotting offers unique advantages in its ability to examine RNA-protein interactions, it also comes with certain limitations, including complexity, time consumption, and the need for optimization. Researchers should carefully weigh these factors when deciding whether Northwestern blotting is the most suitable approach for their research goals.
Furthermore, modern alternatives, such as RNA immunoprecipitation (RIP), CLIP-Seq, RNA-Seq, EMSA, and mass spectrometry, have expanded the toolkit available for the study of RNA-protein interactions. These alternatives often offer higher sensitivity, throughput, and quantitative capabilities, making them suitable for a broader range of applications.
Possible References Used