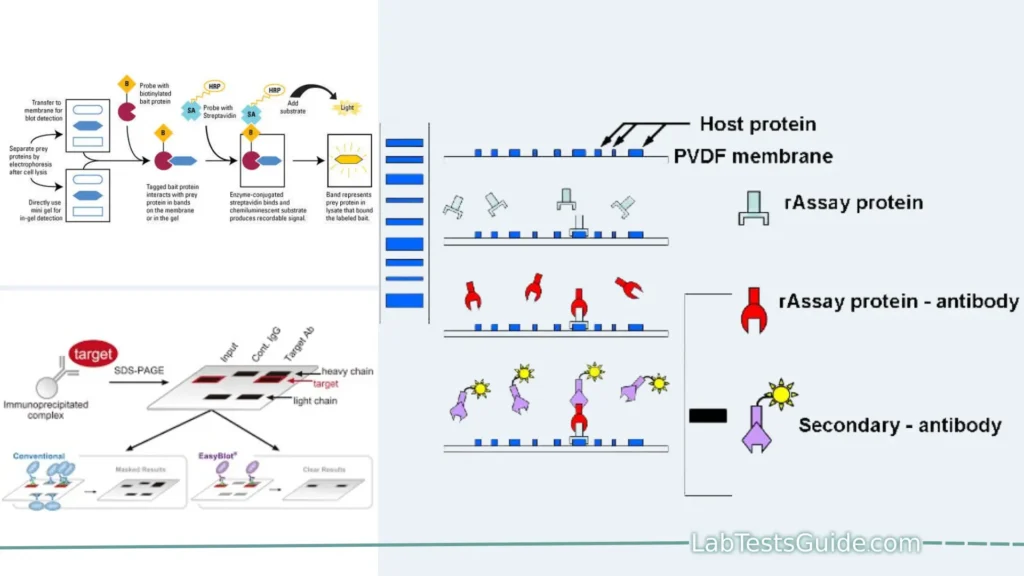
Far-western blotting is a molecular biological technique used to detect protein-protein interactions in vitro. It is similar to western blotting, but instead of using an antibody probe to detect a protein of interest, far-western blotting uses a non-antibody probe such as a tagged protein or a small molecule. This makes far-western blotting a more versatile technique, as it can be used to detect protein-protein interactions that would not be possible to detect with antibodies.
Key points of Far-Western Blotting:
Far-Western blotting is a specialized technique used to study protein-protein interactions. Here are 17 key points to understand about Far-Western blotting:
- Protein Interaction Analysis: Far-Western blotting is a method for investigating and characterizing protein-protein interactions.
- Variation of Western Blotting: It is derived from the traditional Western blotting technique but is focused on detecting direct protein-protein interactions.
- Protein Separation: Like in Western blotting, proteins are first separated based on their size using SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis).
- Transfer to a Membrane: After separation, proteins are transferred onto a membrane, typically made of nitrocellulose or PVDF.
- Non-Antibody Probe: In Far-Western blotting, a non-antibody protein probe is used instead of antibodies. This probe is usually labeled for detection.
- Direct Binding: The probe is incubated with the membrane containing the immobilized proteins, and it directly binds to its interacting partner(s).
- Washing Steps: After incubation, the membrane is washed to remove unbound or nonspecifically bound probe molecules.
- Detection Methods: The presence of protein-protein interactions is detected using various methods, depending on the label used, such as chemiluminescence, fluorescence, or autoradiography.
- Focus on Direct Interactions: Far-Western blotting primarily detects direct interactions between proteins, making it valuable for studying signaling pathways.
- Limitations: It is limited to detecting direct interactions, and proteins are denatured during SDS-PAGE, which may affect native protein confirmation.
- Suitable for Linear Peptide Motifs: Far-Western blotting is most effective for studying domains that bind to short, linear peptide motifs.
- Probe Selection: Choosing the right probe is crucial for successful Far-Western blotting experiments. Probes should be easy to purify and of suitable size.
- Applications: Far-Western blotting is used to detect protein binding partners and assess post-translational modifications in proteins.
- GST Fusion Proteins: Probes are often fused with glutathione S-transferases (GST) to facilitate purification and detection using anti-GST antibodies.
- Optimization: The quality and activity of the probe and binding conditions should be optimized for reliable results.
- Positive and Negative Controls: Including positive and negative controls is essential to validate the results and minimize false positives.
- Stripping and Reprobing: While fresh membranes are preferred, in some cases, membranes can be stripped and reprobed to conserve resources, but this may result in signal loss and increased background noise.
Defination of Far-Western Blotting:
Far-Western blotting is a laboratory technique used to study and characterize protein-protein interactions by detecting direct physical associations between proteins immobilized on a membrane and a labeled non-antibody protein probe.
Background and Significance:
Background:
- Introduction to the Field: Begin by introducing the broader field of study to give readers a sense of the subject matter.
- Historical Context: Discuss key historical developments and previous research that have contributed to the current state of knowledge in the field.
- Gaps in Knowledge: Identify gaps or limitations in the existing literature that your research aims to address. Explain why these gaps are important.
- Relevance: Explain the relevance of your research within the broader context of science, society, or a specific field. Why is it important to study this topic?
- Research Question/Hypothesis: Clearly state your research question or hypothesis that your study aims to answer or test.
Significance:
- Scientific Contribution: Explain how your research will contribute to the advancement of knowledge in your field. What new insights or discoveries do you hope to make?
- Practical Implications: Discuss the practical applications or real-world significance of your research. How might your findings be used to solve practical problems or improve a particular situation?
- Potential Benefits: Highlight the potential benefits that may arise from your research, such as improved technologies, better understanding of diseases, or policy recommendations.
- Impact on Society: Consider the broader societal impact of your research. How might it benefit society or address pressing issues?
- Innovation: If your research involves novel approaches, methodologies, or technologies, emphasize the innovative aspects and how they might revolutionize the field.
- Interdisciplinary Relevance: Explain if your research has interdisciplinary relevance, potentially bridging gaps between different fields or fostering collaborations.
- Addressing Global Challenges: If applicable, discuss how your research aligns with global challenges, such as sustainability, public health, or climate change.
- Ethical Considerations: Briefly mention any ethical considerations associated with your research and how they will be addressed.
- Future Directions: Suggest potential future directions or applications that could build upon your research.
- Conclusion: Summarize the overall significance of your research in a concise and compelling manner, leaving a lasting impression on the reader.
Purpose of Far-Western Blotting:
- Detect Protein-Protein Interactions: Identify and characterize direct physical interactions between proteins.
- Study Signaling Pathways: Explore signaling networks and understand how proteins communicate within cells.
- Assess Binding Specificity: Determine the specificity and affinity of protein-protein interactions.
- Investigate Post-Translational Modifications: Examine how post-translational modifications influence protein interactions.
- Validate Interactions: Confirm protein binding partners following immunoprecipitation or other assays.
- Explore Molecular Mechanisms: Gain insights into the molecular mechanisms underpinning cellular processes.
- Advance Biological Research: Contribute to the broader field of molecular biology and cell signaling by expanding our understanding of protein interactions.
Applications of Far-Western Blotting:
- Identify Protein Binding Partners: Detect and confirm specific protein-protein interactions, shedding light on cellular processes.
- Study Signaling Pathways: Analyze signaling networks by investigating direct protein interactions in complex pathways.
- Assess Post-Translational Modifications: Investigate how modifications like phosphorylation impact protein interactions.
- Characterize Protein Domains: Determine which protein domains are responsible for binding to specific motifs.
- Drug Discovery: Screen for potential drug targets and assess the effects of compounds on protein interactions.
- Functional Proteomics: Understand the roles of proteins in cellular functions and disease pathways.
- Diagnosis and Biomarker Discovery: Identify potential disease biomarkers by examining altered protein interactions.
- Cell Signaling Research: Explore the intricate signaling networks within cells to unravel disease mechanisms.
- Molecular Biology: Advance the field by providing insights into molecular interactions and regulatory mechanisms.
Principles of Far-Western Blotting:
The principles of Far-Western blotting involve the detection of protein-protein interactions using a non-antibody protein probe. Here’s a detailed breakdown of the principles:
- Protein Separation: Far-Western blotting begins with the separation of proteins from a biological sample using SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis). This process separates proteins based on their molecular weight.
- Transfer to a Membrane: After separation, the proteins are transferred from the gel onto a membrane, typically made of nitrocellulose or PVDF (Polyvinylidene Fluoride). This membrane serves as the substrate for subsequent probing.
- Non-Antibody Protein Probe: In contrast to traditional Western blotting, where antibodies are used for detection, Far-Western blotting employs a non-antibody protein probe. This probe is a purified protein of interest, typically labeled for detection.
- Protein-Protein Interaction: The labeled protein probe is incubated with the membrane containing the immobilized proteins. During this incubation, the probe directly binds to its interacting partner(s) among the immobilized proteins.
- Washing Steps: Following incubation, the membrane undergoes thorough washing steps to remove any unbound or nonspecifically bound probe molecules.
- Detection: The presence of protein-protein interactions is detected by visualizing the labeled probe. The choice of detection method depends on the label used, which could be a fluorescent tag, chemiluminescence, or autoradiography in the case of a radioactive label.
- Direct Interaction Focus: Far-Western blotting is primarily used to detect direct interactions between proteins. It is particularly valuable for studying signaling pathways and protein complexes.
- Limitations: Far-Western blotting has limitations, including the denaturation of proteins during SDS-PAGE, which may affect the confirmation of some proteins. It is most effective for studying domains that bind to short, linear peptide motifs.
- Probe Selection: Choosing the appropriate probe is critical. The probe should be easy to purify and of a suitable size. Probes are often fused with tags like glutathione S-transferases (GST) for purification and detection.
- Optimization: Ensuring the quality and activity of the probe and optimizing binding conditions are essential for reliable results.
- Positive and Negative Controls: Including positive and negative controls in experiments helps validate the results and minimize false positives.
Procedure for Far-Western Blotting:
- Protein Separation: Separate proteins using SDS-PAGE.
- Transfer to a Membrane: Transfer the proteins from the gel onto a nitrocellulose or PVDF membrane.
- Blocking: Block the membrane to prevent nonspecific binding.
- Probe Incubation: Incubate the membrane with a labeled non-antibody protein probe.
- Washing: Wash the membrane to remove unbound or nonspecifically bound probes.
- Detection: Visualize protein-protein interactions by detecting the labeled probe using appropriate methods (e.g., chemiluminescence, fluorescence, or autoradiography).
- Analysis: Analyze the results to identify and characterize protein interactions.
- Controls: Include positive and negative controls for validation.
- Interpretation: Interpret the data to draw conclusions about protein interactions.
Materials and Reagents:
Materials and reagents used in Far-Western blotting experiments typically include a range of laboratory supplies and chemicals. Here’s a list of common materials and reagents:
Materials:
- Protein samples: These can be whole cell lysates, purified proteins, or native/denatured proteins, depending on the experiment’s objectives.
- SDS-PAGE Gel: To separate proteins based on size.
- Nitrocellulose or PVDF Membrane: For transferring proteins from the gel.
- Electrophoresis apparatus: To perform SDS-PAGE.
- Membrane transfer apparatus: To transfer proteins from the gel to the membrane.
- Western blotting apparatus: For the blotting process.
- Blocking agent: Such as non-fat milk or bovine serum albumin (BSA) to prevent nonspecific binding.
- Non-antibody protein probe: A purified protein of interest, typically labeled for detection.
- Washing buffer: To remove unbound or nonspecifically bound probes.
- Detection reagents: Depending on the label used, these can include chemiluminescent substrates, fluorescent probes, or autoradiography materials.
- Gel documentation system: For capturing images of the blots.
Reagents:
- SDS-PAGE running buffer: A buffer solution for gel electrophoresis.
- Transfer buffer: To facilitate the transfer of proteins from the gel to the membrane.
- Blocking buffer: To block nonspecific binding sites on the membrane.
- Antibodies (optional): Specific antibodies may be used for additional confirmation of interactions.
- Protein markers: For molecular weight determination.
- Detergents: Such as Triton X-100 or Tween-20 for washing steps.
- Reagents for gel staining: Coomassie Blue or silver stain.
- Chemiluminescent substrate: If using chemiluminescence for detection.
- Fluorescent probes: For fluorescence-based detection.
- Radioactive materials (if necessary): Such as radiolabeled probes for autoradiography.
- Ethidium bromide (EtBr): For visualizing DNA markers in the gel (if DNA probes are used).
- Buffers: Various buffers such as Tris-buffered saline (TBS), phosphate-buffered saline (PBS), and others for sample preparation and washes.
- Gel-fixing solution: To stabilize proteins in the gel before transfer.
- Antibodies (if used): Specific antibodies for probing protein interactions.
- Protease and phosphatase inhibitors: To preserve protein integrity during sample preparation.
- Chemicals for labeling the probe (if needed): Such as biotin, radioactive isotopes, or fluorescent dyes.
- Chemicals for stripping and reprobing (if necessary): Such as acidic or SDS-containing stripping buffers.
- Molecular biology-grade water: For preparing solutions and buffers.
- Laboratory equipment: This includes pipettes, microcentrifuges, water baths, and gel electrophoresis chambers.
Step-by-Step Protocol:
A step-by-step protocol for Far-Western blotting is outlined below. Please note that this is a generalized protocol, and specific details may vary depending on the particular experiment and the equipment and reagents available in your laboratory. Always follow the manufacturer’s instructions for specific reagents.
Materials and Reagents:
- Protein samples (e.g., whole cell lysates)
- SDS-PAGE gel and running buffer
- Nitrocellulose or PVDF membrane
- Electrophoresis and blotting apparatus
- Blocking buffer (e.g., 5% non-fat milk in TBST)
- Non-antibody protein probe (purified and labeled)
- TBST buffer (Tris-buffered saline with Tween-20)
- Detection reagents (e.g., chemiluminescent substrate)
- Gel documentation system
- Antibodies (optional)
- Protein markers
- Detergents (e.g., Tween-20)
- Reagents for gel staining (e.g., Coomassie Blue)
- Chemiluminescent substrate
- Buffers (e.g., TBS, PBS)
- Gel-fixing solution
- Protease and phosphatase inhibitors
- Chemicals for labeling the probe (if needed)
- Stripping and reprobing chemicals (if necessary)
Protocol:
- Prepare Protein Samples:
- Extract and prepare protein samples (e.g., whole cell lysates) from your experimental system.
- Add protease and phosphatase inhibitors to prevent protein degradation and phosphorylation changes.
- Quantify protein concentrations using a suitable method, such as the Bradford assay.
- Prepare SDS-PAGE Gel:
- Cast and assemble an SDS-PAGE gel according to the desired protein size range.
- Load protein samples, along with protein markers, into the gel wells.
- Run the gel at the appropriate voltage and time until proteins are separated.
- Transfer Proteins to Membrane:
- Set up a transfer apparatus and transfer the separated proteins from the gel to a nitrocellulose or PVDF membrane using transfer buffer.
- Verify successful transfer by staining the gel with Coomassie Blue or using a suitable gel documentation system.
- Blocking:
- Incubate the membrane in blocking buffer (e.g., 5% non-fat milk in TBST) to block nonspecific binding sites for 1-2 hours at room temperature or overnight at 4°C.
- Probe Incubation:
- Prepare the non-antibody protein probe by thawing it on ice.
- Label the probe (if necessary) and dilute it in blocking buffer to the desired concentration.
- Incubate the blocked membrane with the labeled probe for 1-2 hours at room temperature or overnight at 4°C with gentle shaking.
- Washing:
- Wash the membrane with TBST buffer several times (3-5 times for 5-10 minutes each) to remove unbound or nonspecifically bound probes.
- Detection:
- Use appropriate detection reagents (e.g., chemiluminescent substrate) to visualize the presence of the labeled probe on the membrane.
- Capture images of the blot using a gel documentation system or X-ray film.
- Optional: Antibody Confirmation:
- If desired, confirm the detected interactions with specific antibodies by performing a Western blot using primary and secondary antibodies.
- Analysis:
- Analyze the results to identify and characterize protein interactions. Compare with positive and negative controls.
- Optional: Stripping and Reprobing:
- If you wish to probe the same membrane with a different probe, you can strip the membrane using appropriate stripping and reprobing chemicals and repeat steps 4-9.
- Data Interpretation:
- Interpret the data, draw conclusions about protein interactions, and document the results in your research records.
- Dispose of Waste:
- Properly dispose of hazardous materials and waste generated during the experiment.
This protocol provides a general overview of the steps involved in Far-Western blotting. Adapt the details to suit your specific experiment and reagents.
Result Interpretation:
Interpreting the results of a Far-Western blotting experiment involves analyzing the visualized protein-protein interactions on the membrane. Here’s a step-by-step guide on how to interpret Far-Western blotting results:
- Image Visualization:
- Begin by examining the developed membrane using a gel documentation system, X-ray film, or other appropriate detection methods.
- You should see specific spots or bands on the membrane corresponding to the protein-protein interactions.
- Positive Controls:
- Check the results against positive controls. Positive controls should show clear and expected interactions. This confirms the validity of your experimental setup.
- Negative Controls:
- Evaluate negative controls to ensure there are no false-positive interactions. Negative controls should ideally show no interactions.
- Specificity of Interactions:
- Determine the specificity of the interactions. Are the detected interactions specific to the labeled probe protein, or are there nonspecific interactions?
- Specific interactions should align with your expectations based on prior knowledge.
- Relative Affinity:
- Assess the intensity of the spots or bands. The intensity can indicate the relative affinity of the protein-protein interactions. Stronger signals suggest higher affinity.
- Confirmation with Antibodies (Optional):
- If antibodies were used for confirmation, compare the results of the Far-Western blot with those of the Western blot.
- Consistent results between the two blots provide additional confidence in the detected interactions.
- Size and Mobility:
- Consider the size and mobility of the detected interactions. Are they consistent with the expected sizes of the interacting proteins?
- Be aware of any post-translational modifications that could affect protein mobility.
- Reproducibility:
- Replicate the experiment if possible to ensure the reproducibility of the results. Consistency in results across replicates strengthens the validity of the findings.
- Data Quantification (Optional):
- If quantification is required, use image analysis software to measure the intensity of spots or bands. This can provide quantitative data on the interactions.
- Comparison with Previous Studies:
- Compare your results with previous research or literature to see if they align with established knowledge or contribute new insights.
- Data Documentation:
- Document all findings, including positive and negative results, in your research records. Accurate record-keeping is essential for future reference and publication.
- Statistical Analysis (Optional):
- If applicable, perform statistical analysis to assess the significance of differences in interaction intensities or patterns between experimental groups.
- Discussion and Conclusion:
- Discuss the implications of your findings. What do the detected interactions reveal about the biology or signaling pathways you are studying?
- Draw conclusions based on the results and discuss their significance in the context of your research objectives.
- Reporting:
- If the results are part of a research paper or report, present them clearly and concisely in figures and tables, and provide a detailed description of the interpretations in the results section.
Troubleshooting and Tips:
- Weak or No Signal:
- Check the probe concentration; it may need to be increased.
- Verify the quality and activity of the probe.
- Ensure proper blocking to minimize nonspecific binding.
- Optimize wash steps to reduce background.
- High Background:
- Adjust blocking conditions; try different blocking agents or concentrations.
- Ensure thorough washing to remove unbound probes.
- Reduce antibody concentration if antibodies are used for detection.
- Non-Specific Bands:
- Use a more specific probe if available.
- Optimize probe dilution and incubation time.
- Consider using secondary antibodies with higher specificity.
- Smearing on the Gel:
- Ensure the gel is properly cast and free of air bubbles.
- Verify that the gel electrophoresis conditions are appropriate for your samples.
- Uneven Protein Transfer:
- Check for air bubbles or incomplete wetting of the membrane.
- Ensure proper assembly of the transfer apparatus.
- Low Signal-to-Noise Ratio:
- Optimize the exposure time when capturing blot images.
- Use high-quality detection reagents for improved sensitivity.
- Be cautious of overexposure, which can lead to signal saturation.
- Protein Denaturation:
- Consider native gel electrophoresis if studying native protein interactions.
- Optimize sample preparation to minimize denaturation.
- Problems with Antibody Confirmation (if used):
- Ensure antibody specificity and proper dilutions.
- Perform a Western blot with the same samples to verify interactions.
- Inconsistent Results:
- Maintain consistent experimental conditions and equipment settings.
- Repeat experiments and compare results for consistency.
- Limited Binding Specificity:
- Understand that Far-Western blotting is best suited for linear peptide motif interactions; it may not detect all types of protein-protein interactions.
- Controls and Validation:
- Always include positive and negative controls to validate results.
- Verify the quality and activity of the probe.
- Documentation and Record-Keeping:
- Keep thorough records of all experimental details for troubleshooting and future reference.
Advantages and Disadvantages of Far-Western Blotting:
Advantages of Far-Western Blotting:
- Protein-Protein Interaction Analysis: Allows direct assessment of protein-protein interactions, providing insights into signaling networks.
- High Specificity: Can detect specific interactions between modular domains and short linear peptide motifs.
- No Antibodies Required: Eliminates the need for specific antibodies, making it suitable for non-antibody protein probes.
- No Cross-Reactivity: Minimizes cross-reactivity concerns often encountered in traditional Western blotting.
- Versatility: Applicable to a wide range of protein samples, including purified proteins, whole cell lysates, and denatured proteins.
- Quantitative Potential: Can provide quantitative data through image analysis.
Disadvantages of Far-Western Blotting:
- Limited to Specific Interactions: Only detects interactions between modular domains and short linear peptide motifs, limiting its scope.
- Denaturation Requirement: Proteins in the sample are typically denatured, which may not represent native interactions.
- Limited Sensitivity: Detection may be less sensitive compared to some other protein-protein interaction assays.
- Technical Expertise Required: Requires expertise in protein purification, electrophoresis, and blotting techniques.
- Time-Consuming: The procedure involves multiple steps and can be time-consuming.
- Validation Needed: Results should be confirmed by additional assays or controls to validate interactions.
Limitations of Far-Western Blotting:
- Limited to Specific Interactions: Far-Western blotting primarily detects interactions between modular domains and short linear peptide motifs, restricting its applicability to specific types of protein-protein interactions.
- Denaturation Requirement: Proteins in the sample are typically denatured, which may not accurately represent native interactions in a cellular context.
- Limited Sensitivity: Detection sensitivity may be lower compared to some other protein-protein interaction assays, potentially missing weak interactions.
- Technical Expertise Required: Performing Far-Western blotting requires expertise in protein purification, electrophoresis, and blotting techniques, making it less accessible to researchers without specialized training.
- Time-Consuming: The procedure involves multiple steps and can be time-consuming, which may not be suitable for high-throughput studies.
- Validation Needed: Results should be confirmed by additional assays or controls to validate the detected interactions, adding complexity to the experimental workflow.
- Protein Denaturation: The denaturation of proteins during electrophoresis may not preserve the native conformation and interactions of proteins.
- Limited Applicability: Far-Western blotting is best suited for studying specific types of interactions and may not be applicable to all protein-protein interaction studies.
Variations and Modern Alternatives:
Variations of Far-Western Blotting:
- Protein Microarrays: Protein microarrays allow for high-throughput screening of protein-protein interactions. Proteins are spotted on a solid support, and their interactions can be probed using labeled proteins or antibodies.
- Peptide Arrays: Similar to protein microarrays, peptide arrays contain short peptide sequences immobilized on a solid surface. They are used to screen for interactions with specific peptide motifs.
- Native Far-Western Blotting: In native Far-Western blotting, proteins are maintained in their native conformation, reducing the denaturation associated with traditional Far-Western blotting. It is suitable for studying native interactions.
Modern Alternatives to Far-Western Blotting:
- Yeast Two-Hybrid (Y2H) Assay: Y2H allows for the detection of protein-protein interactions in vivo by using a genetic reporter system in yeast cells. It is suitable for high-throughput screening.
- Co-Immunoprecipitation (Co-IP): Co-IP enables the isolation of protein complexes from cell lysates using specific antibodies. It is widely used to study endogenous protein-protein interactions.
- Fluorescence Resonance Energy Transfer (FRET): FRET relies on the proximity-dependent transfer of energy between fluorophores attached to interacting proteins. It provides real-time monitoring of interactions in live cells.
- Proximity Ligation Assay (PLA): PLA uses antibodies conjugated with DNA probes to detect protein interactions. When proteins of interest are in close proximity, DNA probes hybridize, and the signal can be visualized.
- BioID and APEX: These proximity-based labeling techniques use fusion proteins to label interacting partners in living cells. They are particularly useful for identifying transient or weak interactions.
- Mammalian Two-Hybrid (M2H) Assay: Similar to Y2H but performed in mammalian cells, M2H allows for the detection of protein-protein interactions in a more physiologically relevant context.
- Chemical Cross-Linking Mass Spectrometry (XL-MS): XL-MS identifies protein interactions by cross-linking proteins in situ and analyzing the cross-linked peptides by mass spectrometry. It provides structural information about interactions.
- Bimolecular Fluorescence Complementation (BiFC): BiFC relies on the reconstitution of a split fluorophore when interacting proteins come into close proximity. It allows for the visualization of interactions in living cells.
Comparison of Far-Western Blotting with Modern Techniques:
Here’s a comparison table of Far-Western blotting with modern techniques used for studying protein-protein interactions:
| Aspect | Far-Western Blotting | Yeast Two-Hybrid (Y2H) | Co-Immunoprecipitation (Co-IP) | Fluorescence Resonance Energy Transfer (FRET) | Proximity Ligation Assay (PLA) | BioID and APEX | Cross-Linking Mass Spectrometry (XL-MS) | Bimolecular Fluorescence Complementation (BiFC) |
|---|---|---|---|---|---|---|---|---|
| Detection Principle | Protein-protein interactions based on probe binding | Genetic reporter system based on yeast growth | Protein complexes isolated using antibodies | Energy transfer between fluorophores | DNA probes hybridization upon proximity | Proximity-based labeling in living cells | Cross-linking followed by mass spectrometry | Reconstitution of a split fluorophore upon interaction |
| Sensitivity | Moderate to high | High | High | High | High | High | High | High |
| Specificity | High | Moderate to high | High | High | High | High | High | High |
| Throughput | Moderate | Low | Low to moderate | Low to high | Moderate | Low to moderate | Low to high | Moderate |
| Native Interaction Study | Limited | No | Yes | Yes | Yes | Yes | Yes | Limited |
| Suitable for Endogenous Proteins | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Limited |
| Real-time Monitoring (Live Cells) | No | No | No | Yes | Yes | Yes | No | Yes |
| Structural Information | No | No | No | Limited | No | No | Yes | Limited |
| Ease of Use | Moderate | Moderate | Moderate | Moderate to high | Moderate | Moderate | High | Moderate |
| Required Expertise | Protein purification and blotting | Molecular biology and yeast handling | Molecular biology and immunoprecipitation | Fluorescence techniques | Molecular biology and immunoassays | Molecular biology and cell culture | Mass spectrometry | Molecular biology and cell culture |
| High-Throughput Screening | No | Yes | No | No | Yes | Yes | Yes | No |
Please note that the choice of technique should be based on your specific research goals, the nature of the protein-protein interactions you want to study, and the available resources and expertise in your laboratory. Each technique has its advantages and limitations.
FAQs:
Q: What is the primary advantage of Far-Western blotting compared to traditional Western blotting?
A: Far-Western blotting allows for the direct detection of protein-protein interactions without the need for specific antibodies, which is a key advantage over traditional Western blotting.
Q: Can Far-Western blotting be used to study protein interactions in live cells?
A: No, Far-Western blotting is typically performed on denatured protein samples and is not suitable for studying interactions in live cells. Techniques like FRET and BiFC are better suited for real-time monitoring of interactions in living cells.
Q: Are there any limitations to the types of protein interactions that Far-Western blotting can detect?
A: Yes, Far-Western blotting is primarily designed to detect interactions between modular domains and short linear peptide motifs. It may not be suitable for studying all types of protein-protein interactions.
Q: What is the advantage of using native Far-Western blotting over the traditional method?
A: Native Far-Western blotting preserves the native conformation of proteins, making it more suitable for studying interactions in a physiological context, but it may be less sensitive than the traditional denaturing method.
Q: Is Far-Western blotting suitable for high-throughput studies?
A: Far-Western blotting is not typically considered a high-throughput technique due to its multi-step process and the need for specialized expertise. Other methods like yeast two-hybrid assays or proximity ligation assays may be more suitable for high-throughput studies.
Q: What are some common controls used in Far-Western blotting experiments?
A: Common controls in Far-Western blotting include using known interacting proteins as positive controls, performing Far-Western blotting without the probe as a negative control, and validating results with additional assays such as co-immunoprecipitation.
Q: Can Far-Western blotting provide quantitative data on protein interactions?
A: Yes, Far-Western blotting can provide quantitative data through image analysis of the intensity of the bands formed on the blot. However, quantification may be less accurate than some other techniques like enzyme-linked immunosorbent assays (ELISA).
Q: Is Far-Western blotting suitable for studying endogenous protein interactions in complex cellular samples?
A: Far-Western blotting can be used to study endogenous protein interactions in complex samples, but it requires careful sample preparation and optimization to minimize background and ensure specificity.
Q: What are some modern alternatives to Far-Western blotting for studying protein-protein interactions?
A: Modern alternatives include yeast two-hybrid assays, co-immunoprecipitation, fluorescence resonance energy transfer (FRET), proximity ligation assays (PLA), BioID and APEX proximity labeling, cross-linking mass spectrometry (XL-MS), and bimolecular fluorescence complementation (BiFC), among others. The choice depends on specific research goals and experimental conditions.
Conclusion:
In conclusion, Far-Western blotting is a valuable technique for investigating protein-protein interactions. It offers unique advantages such as the ability to detect direct interactions without the need for specific antibodies and the potential for quantitative analysis. However, it also has limitations, including its focus on specific types of interactions and the denaturation of proteins during the process.
Researchers should carefully consider their experimental goals and the nature of the interactions they wish to study when choosing Far-Western blotting or alternative methods. While Far-Western blotting remains a powerful tool, modern techniques like yeast two-hybrid assays, co-immunoprecipitation, and proximity labeling offer additional capabilities and may be better suited for certain applications.
Possible References Used