CLIA vs ECLIA: Chemiluminescence Immunoassay (CLIA) and Electrochemiluminescence Immunoassay (ECLIA) are both types of immunoassay technologies used in clinical laboratories to measure the concentration of specific analytes in patient samples.
Definations:
Defination of CLIA:
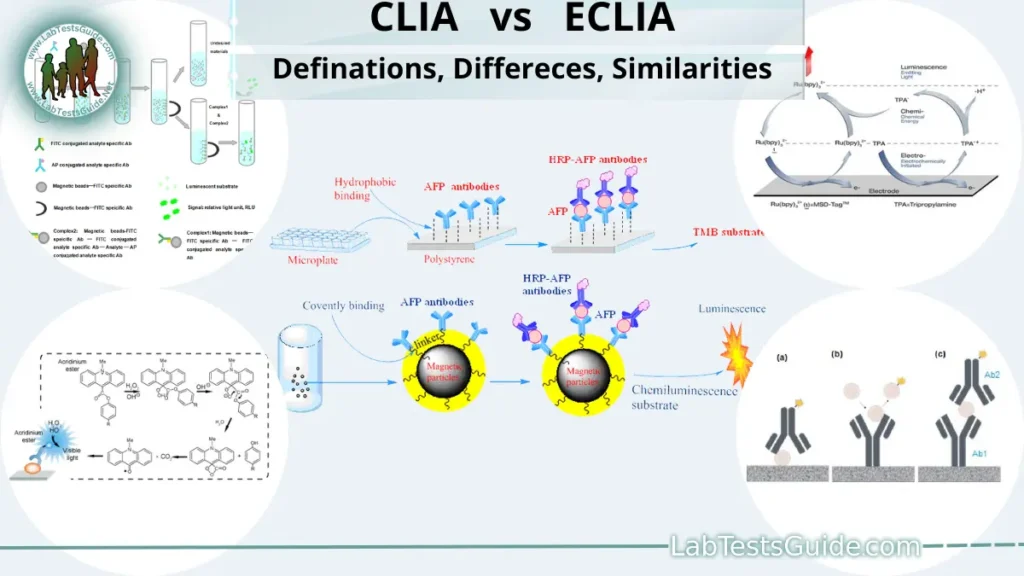
Chemiluminescence Immunoassay (CLIA) is a type of immunoassay that uses a chemical reaction to produce light (luminescence) as a result of the interaction between a labeled molecule (such as an enzyme or a fluorescent molecule) and a specific antigen or antibody present in a biological sample. The intensity of the emitted light is proportional to the concentration of the target analyte, allowing for sensitive and quantitative detection of various substances in clinical diagnostics and research.
Defination of ECLIA:
Electrochemiluminescence Immunoassay (ECLIA) is an immunoassay method that relies on electrochemistry to generate light emission. In ECLIA, the label attached to the analyte or antibody is an electroactive molecule. When a voltage is applied to the solution containing the labeled components, electrochemical reactions occur, leading to the production of light (luminescence). The intensity of the emitted light is proportional to the concentration of the target analyte, making ECLIA a highly sensitive and versatile technique used in clinical and research laboratories for detecting various biomolecules.
Principles:
Principle of CLIA:
CLIA is based on the principle of a chemical reaction between a labeled molecule and a specific antigen or antibody. The labeled molecule is usually an enzyme or a fluorescent molecule conjugated to one of the components involved in the immunoassay. When a biological sample containing the target analyte is introduced to a reagent solution containing the labeled molecule and other necessary components, a reaction occurs between the labeled molecule and the analyte or antibody.
The chemical reaction releases energy in the form of light (luminescence) without the requirement of an external light source. The intensity of the emitted light is directly proportional to the amount of the target analyte present in the sample. A highly sensitive detector measures the luminescent signal, and the concentration of the analyte is determined based on the detected signal.
Principle of ECLIA:
ECLIA operates on the principle of electrochemistry to generate luminescence. Similar to CLIA, ECLIA also involves a labeled molecule (an electroactive molecule) that is conjugated to one of the components of the immunoassay, such as the analyte or antibody. When an electric potential (voltage) is applied to the solution containing the labeled components, an electrochemical reaction occurs at the electrode surface.
The electrochemical reaction induces energy transfer to the electroactive molecule, exciting it to a higher energy state. As the molecule returns to its ground state, it emits light (luminescence). The intensity of the emitted light is directly proportional to the concentration of the target analyte in the sample. An electrochemical detector measures the luminescent signal, and the analyte concentration is quantified based on this signal.
Advantages:
Advantages of CLIA
- Sensitivity: CLIA is highly sensitive, capable of detecting analytes at low concentrations, making it suitable for diagnosing diseases in early stages or monitoring low-abundance biomarkers.
- Speed: CLIA provides rapid results, allowing for quick turnaround times in clinical laboratories and enabling efficient patient care.
- Wide Analyte Range: CLIA can be adapted to detect a broad range of analytes, including hormones, tumor markers, infectious agents, and drugs, making it versatile for various clinical applications.
- Simplicity: The assay procedure for CLIA is relatively simple and straightforward, requiring minimal hands-on time and technical expertise.
- Automation: CLIA can be easily automated, allowing for high-throughput analysis, reducing manual errors, and improving overall laboratory efficiency.
- Stability: Chemiluminescent signals in CLIA are generally stable over time, providing consistent and reliable results during storage and transportation of samples.
Advantages of ECLIA
- High Sensitivity: ECLIA is extremely sensitive, capable of detecting analytes at very low concentrations, enhancing the accuracy of measurement in critical diagnostic situations.
- Wide Dynamic Range: ECLIA has a broad dynamic range, allowing it to quantify both high and low concentrations of analytes without the need for sample dilution.
- Minimal Interference: Electrochemical reactions in ECLIA are less susceptible to interference from other substances present in the sample, improving assay specificity and accuracy.
- Precision: ECLIA offers high precision and reproducibility, providing reliable and consistent results, even in trace-level analyte detection.
- Reduced Sample Volume: ECLIA requires smaller sample volumes, which can be beneficial when dealing with limited or precious samples, especially in pediatric or geriatric patients.
- Less Matrix Effect: ECLIA exhibits a reduced matrix effect, meaning the assay performance is less affected by the composition of the biological sample matrix.
Disadvantages:
Disadvantages of CLIA
- Limited Dynamic Range: CLIA may have limitations in measuring very high concentrations of analytes, as the signal may saturate at higher levels.
- Potential Interference: The chemical reactions involved in CLIA can be susceptible to interference from certain substances present in the sample, leading to inaccurate results.
- Reagent Complexity: Some chemiluminescent assays may require more complex reagents and protocols, which could increase the risk of errors during testing.
- Signal Decay: The luminescent signal in CLIA may decay over time, which can affect the accuracy of results if there are delays in analysis.
- Cost of Reagents: In some cases, the cost of chemiluminescent reagents can be higher compared to other immunoassay methods.
- Operator Expertise: While CLIA is generally considered straightforward, proper training is still essential to ensure accurate and reliable results.
Disadvantages of ECLIA
- Equipment Cost: ECLIA requires specialized electrochemical analyzers, which can be more expensive to acquire and maintain compared to some other immunoassay platforms.
- Technical Complexity: Operating and maintaining the electrochemical analyzer may demand more technical expertise than some other immunoassay methods.
- Limited Availability: ECLIA instruments may not be as widely available as other immunoassay platforms, especially in smaller or resource-limited laboratories.
- Potential Matrix Effects: Although ECLIA is generally less affected by matrix interference, some complex samples may still exhibit matrix effects that can impact assay performance.
- Sample Stability: Certain electroactive labels used in ECLIA may be susceptible to sample stability issues, affecting the accuracy of results over time.
- Electrode Contamination: Contamination of the electrodes can affect the electrochemical reactions and lead to inaccurate measurements.
Limitations:
Here are some limitations of both Chemiluminescence Immunoassay (CLIA) and Electrochemiluminescence Immunoassay (ECLIA):
Limitations of CLIA:
- Cross-Reactivity: CLIA can sometimes exhibit cross-reactivity with structurally similar compounds, leading to potential false-positive or false-negative results.
- Complex Sample Matrix: Samples with complex matrices, such as blood or urine, may contain interfering substances that could affect the accuracy of CLIA results.
- Sensitivity to Environmental Factors: The chemiluminescent reaction in CLIA can be sensitive to environmental conditions like temperature and light, which may impact assay performance and require careful handling of samples and reagents.
- Limited Stability of Reagents: Some CLIA reagents may have a limited shelf life and require proper storage conditions to maintain their stability and reliability.
- Inter-laboratory Variability: Variations in instrumentation, reagents, and procedures across different laboratories can lead to differences in results, making standardization and quality control crucial for accurate comparability.
Limitations of ECLIA
- Complexity of Instrumentation: ECLIA instruments can be relatively complex and may require specialized training to operate and maintain, leading to a higher initial investment and ongoing costs.
- Matrix Interference: Although less prone to matrix interference compared to other immunoassay methods, certain complex samples may still present challenges for ECLIA performance.
- Electrode Maintenance: Regular maintenance and cleaning of the electrodes in ECLIA instruments are essential to ensure accurate and reliable results.
- Limited Assay Development: Some specific analytes may not have commercially available ECLIA assays, limiting its applicability for certain niche or rare biomarkers.
- Sample Volume Requirements: ECLIA may require larger sample volumes compared to other immunoassay methods, which can be a limitation when dealing with limited or precious samples.
- Interference from Anticoagulants: Certain anticoagulants used in blood collection tubes can interfere with the ECLIA reaction, affecting the accuracy of results.
Comparison Between CLIA and ECLIA:
Comparison Between Chemiluminescence Immunoassay (CLIA) and Electrochemiluminescence Immunoassay (ECLIA):
- Principle:
- CLIA uses a chemical reaction between a labeled molecule and the analyte to produce luminescence.
- ECLIA relies on electrochemistry to generate luminescence through an electrochemical reaction at the electrode surface.
- Sensitivity:
- Both CLIA and ECLIA are highly sensitive, capable of detecting analytes at low concentrations.
- ECLIA generally offers higher sensitivity due to the nature of electrochemical reactions, allowing for detection of even lower analyte concentrations.
- Dynamic Range:
- CLIA may have limitations in measuring very high analyte concentrations as the signal may saturate.
- ECLIA has a broader dynamic range and can quantify both high and low analyte concentrations without the need for sample dilution.
- Interference:
- CLIA may be susceptible to interference from certain substances in the sample, leading to potential inaccuracies.
- ECLIA exhibits reduced interference from sample matrix components, enhancing assay specificity and accuracy.
- Equipment:
- CLIA generally requires standard laboratory equipment and may not demand specialized analyzers.
- ECLIA requires specialized electrochemical analyzers, which can be more expensive to acquire and maintain.
- Sample Volume:
- CLIA typically requires smaller sample volumes, which can be advantageous when dealing with limited or precious samples.
- ECLIA may require larger sample volumes compared to CLIA.
- Technical Expertise:
- Both CLIA and ECLIA require some level of technical expertise, but ECLIA may demand more specialized training for operating and maintaining the electrochemical analyzer.
- Automation and Throughput:
- Both CLIA and ECLIA can be automated, but CLIA may have an advantage in terms of simplicity and ease of automation for high-throughput analysis.
- Cost:
- CLIA is generally considered more cost-effective than ECLIA, especially regarding equipment and reagents.
- Availability:
- CLIA assays are widely available, and a broad range of commercially available kits exist.
- ECLIA instruments and assays may be less readily available, particularly in smaller or resource-limited laboratories.
Differences Between CLIA and ECLIA:
Here are 14 differences between Chemiluminescence Immunoassay (CLIA) and Electrochemiluminescence Immunoassay (ECLIA):
- Detection Mechanism:
- CLIA: Uses a chemical reaction between a labeled molecule and the target analyte to produce luminescence.
- ECLIA: Relies on electrochemistry to generate luminescence through an electrochemical reaction at the electrode surface.
- Sensitivity:
- CLIA: Highly sensitive, capable of detecting low analyte concentrations.
- ECLIA: Generally offers higher sensitivity, detecting even lower analyte concentrations due to the nature of electrochemical reactions.
- Dynamic Range:
- CLIA: May have limitations in measuring very high analyte concentrations as the signal may saturate.
- ECLIA: Has a broader dynamic range and can quantify both high and low analyte concentrations without the need for sample dilution.
- Interference:
- CLIA: May be susceptible to interference from certain substances in the sample, leading to potential inaccuracies.
- ECLIA: Exhibits reduced interference from sample matrix components, enhancing assay specificity and accuracy.
- Equipment:
- CLIA: Generally requires standard laboratory equipment and may not demand specialized analyzers.
- ECLIA: Requires specialized electrochemical analyzers, which can be more expensive to acquire and maintain.
- Sample Volume:
- CLIA: Typically requires smaller sample volumes, advantageous for limited or precious samples.
- ECLIA: May require larger sample volumes compared to CLIA.
- Technical Expertise:
- CLIA: Requires some technical expertise, but generally considered simpler than ECLIA.
- ECLIA: Demands more specialized training for operating and maintaining the electrochemical analyzer.
- Automation and Throughput:
- CLIA: Can be automated, but may have an advantage in simplicity for high-throughput analysis.
- ECLIA: Also automatable, but complexity may influence high-throughput capabilities.
- Cost:
- CLIA: Generally considered more cost-effective, especially regarding equipment and reagents.
- ECLIA: Can be more expensive due to specialized equipment and reagents.
- Availability:
- CLIA: Assays are widely available with a broad range of commercially available kits.
- ECLIA: Instruments and assays may be less readily available, particularly in smaller or resource-limited laboratories.
- Stability of Reagents:
- CLIA: Some reagents may have a limited shelf life, requiring proper storage.
- ECLIA: Reagents are typically more stable over time.
- Electrode Maintenance:
- CLIA: No specific electrode maintenance required.
- ECLIA: Regular maintenance and cleaning of electrodes are essential for accurate results.
- Signal Generation:
- CLIA: Luminescent signal generated through a chemical reaction.
- ECLIA: Luminescent signal generated through electrochemical reactions.
- Matrix Effect:
- CLIA: May be more prone to matrix interference in complex samples.
- ECLIA: Exhibits reduced matrix effects, improving assay performance in complex samples.
Table of Differences:
| Aspect | Chemiluminescence Immunoassay (CLIA) | Electrochemiluminescence Immunoassay (ECLIA) |
|---|---|---|
| Detection Mechanism | Chemical reaction between labeled molecule and target analyte | Electrochemical reaction at the electrode surface |
| Sensitivity | Highly sensitive, detects low analyte concentrations | Generally higher sensitivity, detects lower analyte concentrations |
| Dynamic Range | Limited for very high analyte concentrations | Broader dynamic range, quantifies both high and low concentrations |
| Interference | Susceptible to interference from certain substances | Reduced interference from sample matrix components |
| Equipment | Standard laboratory equipment | Requires specialized electrochemical analyzers |
| Sample Volume | Requires smaller sample volumes | May require larger sample volumes |
| Technical Expertise | Generally simpler, requires basic training | Demands more specialized training for the analyzer |
| Automation and Throughput | Automatable, efficient for high-throughput analysis | Also automatable, complexity may influence throughput |
| Cost | Generally cost-effective | Can be more expensive due to specialized equipment |
| Availability | Widely available with various commercial kits | Instruments and assays may be less readily available |
| Stability of Reagents | Some reagents may have limited shelf life | Reagents are typically more stable over time |
| Electrode Maintenance | Not applicable, no specific electrode maintenance | Regular maintenance and cleaning required |
| Signal Generation | Luminescence through a chemical reaction | Luminescence through electrochemical reactions |
| Matrix Effect | Prone to matrix interference in complex samples | Reduced matrix effects, improved assay performance |
Similarities Between CLIA and ECLIA:
Here are 11 similarities between Chemiluminescence Immunoassay (CLIA) and Electrochemiluminescence Immunoassay (ECLIA):
- Immunoassay Technique: Both CLIA and ECLIA are types of immunoassays that utilize specific antigen-antibody interactions for the detection and quantification of analytes in biological samples.
- Labeling Strategy: In both CLIA and ECLIA, a labeled molecule (e.g., an enzyme or a fluorescent molecule) is used to bind to the target analyte or antibody, enabling the generation of luminescence during the detection process.
- Luminescent Signal: Both methods rely on luminescent signals for analyte quantification. The emitted light is proportional to the concentration of the target analyte in the sample.
- High Sensitivity: Both CLIA and ECLIA are highly sensitive techniques capable of detecting low concentrations of analytes, making them suitable for diagnosing diseases in early stages or detecting low-abundance biomarkers.
- Quantitative Analysis: Both methods allow for quantitative analysis, providing accurate measurements of analyte concentrations in the sample.
- Specificity: CLIA and ECLIA offer high specificity by using specific antigen-antibody interactions, ensuring minimal cross-reactivity with other substances in the sample.
- Clinical Applications: Both CLIA and ECLIA are widely used in clinical diagnostics for various applications, including measuring hormones, proteins, tumor markers, infectious agents, and drugs.
- Calibration Curve: Both assays utilize calibration or standard curves with known concentrations of the analyte to convert the measured luminescent signal into quantitative results.
- Laboratory Equipment: While the instrumentation may differ, both methods typically use standard laboratory equipment, including microplate readers or specialized analyzers.
- Automation: Both CLIA and ECLIA can be automated, enabling high-throughput analysis and improving efficiency in clinical laboratories.
- Quality Control: Both methods require proper validation and quality control measures to ensure the accuracy and reliability of results, especially in clinical settings.
Table of Similarities:
| Aspect | Chemiluminescence Immunoassay (CLIA) | Electrochemiluminescence Immunoassay (ECLIA) |
|---|---|---|
| Immunoassay Technique | Both are types of immunoassays | Both are types of immunoassays |
| Labeling Strategy | Labeled molecule binds to target analyte | Labeled molecule binds to target analyte |
| Luminescent Signal | Utilizes luminescent signals for detection | Utilizes luminescent signals for detection |
| High Sensitivity | Highly sensitive for low analyte concentrations | Highly sensitive for low analyte concentrations |
| Quantitative Analysis | Provides quantitative measurement of analyte | Provides quantitative measurement of analyte |
| Specificity | Offers high specificity for target analytes | Offers high specificity for target analytes |
| Clinical Applications | Widely used in clinical diagnostics | Widely used in clinical diagnostics |
| Calibration Curve | Utilizes calibration curves for quantification | Utilizes calibration curves for quantification |
| Laboratory Equipment | Uses standard laboratory equipment | Uses standard laboratory equipment |
| Automation | Capable of automation for high-throughput analysis | Capable of automation for high-throughput analysis |
| Quality Control | Requires proper validation and quality control | Requires proper validation and quality control |
FAQs:
FAQ: What is the difference between CLIA and ECLIA?
Answer: The main difference lies in the detection mechanism. CLIA uses a chemical reaction to produce luminescence, while ECLIA relies on electrochemistry. ECLIA generally offers higher sensitivity and a broader dynamic range compared to CLIA.
FAQ: Which assay is more sensitive, CLIA, or ECLIA?
Answer: ECLIA is generally more sensitive than CLIA due to the nature of electrochemical reactions, allowing for the detection of even lower analyte concentrations.
FAQ: What are the advantages of CLIA and ECLIA over other immunoassay methods?
Answer: Both CLIA and ECLIA offer high sensitivity, quantitative analysis, and specificity. They are also capable of detecting low concentrations of analytes, making them valuable in early disease diagnosis and monitoring.
FAQ: Are CLIA and ECLIA suitable for clinical diagnostics?
Answer: Yes, both CLIA and ECLIA are widely used in clinical diagnostics for measuring various analytes, such as hormones, proteins, infectious agents, and drugs.
FAQ: Can CLIA and ECLIA be automated for high-throughput analysis?
Answer: Yes, both CLIA and ECLIA can be automated, which allows for efficient high-throughput analysis in clinical laboratories.
FAQ: What are the limitations of CLIA and ECLIA?
Answer: CLIA may have limitations in measuring very high analyte concentrations, while ECLIA’s main limitation is the requirement for specialized equipment, which can be more expensive.
FAQ: Are there differences in the cost of running CLIA and ECLIA assays?
Answer: Generally, CLIA is considered more cost-effective than ECLIA due to the availability of standard laboratory equipment and reagents.
FAQ: How do CLIA and ECLIA assays handle sample interference?
Answer: CLIA may be more susceptible to interference from certain substances in the sample, while ECLIA exhibits reduced interference from sample matrix components.
FAQ: Which assay should I choose for my specific clinical application?
Answer: The choice between CLIA and ECLIA depends on the specific requirements of the assay, the analyte of interest, and the available resources in the laboratory. Both methods have their advantages and limitations, so careful consideration is essential for the best fit.
Conclusion:
In conclusion, both Chemiluminescence Immunoassay (CLIA) and Electrochemiluminescence Immunoassay (ECLIA) are powerful immunoassay techniques that use luminescence for sensitive and quantitative detection of analytes. They offer high specificity and are widely used in clinical diagnostics and research. ECLIA generally provides higher sensitivity and a broader dynamic range, while CLIA is more cost-effective. The choice between the two methods depends on specific assay requirements and available resources. Both assays continue to play significant roles in modern healthcare and research.
Possible References Used